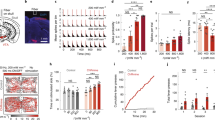
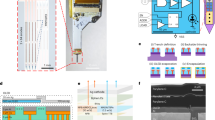
Abstract
Optogenetic methods have been widely used in rodent brains, but remain relatively under-developed for nonhuman primates such as rhesus macaques, an animal model with a large brain expressing sophisticated sensory, motor and cognitive behaviors. To address challenges in behavioral optogenetics in large brains, we developed Opto-Array, a chronically implantable array of light-emitting diodes for high-throughput optogenetic perturbation. We demonstrated that optogenetic silencing in the macaque primary visual cortex with the help of the Opto-Array results in reliable retinotopic visual deficits in a luminance discrimination task. We separately confirmed that Opto-Array illumination results in local neural silencing, and that behavioral effects are not due to tissue heating. These results demonstrate the effectiveness of the Opto-Array for behavioral optogenetic applications in large brains.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
The photometric, thermal and behavioral measurements used in this study are available at https://github.com/RishiRajalingham/NatureMethods2021.
Code availability
The code used to generate behavioral analyses is available at https://github.com/RishiRajalingham/NatureMethods2021.
References
Yizhar, O., Fenno, L. E., Davidson, T. J., Mogri, M. & Deisseroth, K. Optogenetics in neural systems. Neuron 71, 9–34 (2011).
Deisseroth, K. Optogenetics: 10 years of microbial opsins in neuroscience. Nat. Neurosci. 18, 1213–1225 (2015).
Jarvis, S. & Schultz, S. R. Prospects for optogenetic augmentation of brain function. Front. Syst. Neurosci. 9, 157 (2015).
El-Shamayleh, Y. & Horwitz, G. D. Primate optogenetics: progress and prognosis. Proc. Natl Acad. Sci. USA https://doi.org/10.1073/pnas.1902284116 (2019).
Berdyyeva, T. K. & Reynolds, J. H. The dawning of primate optogenetics. Neuron 62, 159–160 (2009).
Galvan, A. et al. Nonhuman primate optogenetics: recent advances and future directions. J. Neurosci. 37, 10894–10903 (2017).
Diester, I. et al. An optogenetic toolbox designed for primates. Nat. Neurosci. 14, 387–397 (2011).
Matsumoto, M., Inoue, K.-I. & Takada, M. Causal role of neural signals transmitted from the frontal eye field to the superior colliculus in saccade generation. Front. Neural Circuits 12, 69 (2018).
Jazayeri, M., Lindbloom-Brown, Z. & Horwitz, G. D. Saccadic eye movements evoked by optogenetic activation of primate V1. Nat. Neurosci. 15, 1368–1370 (2012).
Gerits, A. et al. Optogenetically induced behavioral and functional network changes in primates. Curr. Biol. 22, 1722–1726 (2012).
May, T. et al. Detection of optogenetic stimulation in somatosensory cortex by non-human primates—towards artificial tactile sensation. PLoS ONE 9, e114529 (2014).
Ozden, I. et al. A coaxial optrode as multifunction write-read probe for optogenetic studies in non-human primates. J. Neurosci. Methods 219, 142–154 (2013).
Acker, L., Pino, E. N., Boyden, E. S. & Desimone, R. FEF inactivation with improved optogenetic methods. Proc. Natl Acad. Sci. USA 113, E7297–E7306 (2016).
Dai, J. et al. Modified toolbox for optogenetics in the nonhuman primate. Neurophotonics 2, 031202 (2015).
Sileo, L. et al. Tapered fibers combined with a multi-electrode array for optogenetics in mouse medial prefrontal cortex. Front. Neurosci. 12, 771 (2018).
Ruiz, O. et al. Optogenetics through windows on the brain in the nonhuman primate. J. Neurophysiol. 110, 1455–1467 (2013).
Chernov, M. M., Friedman, R. M., Chen, G., Stoner, G. R. & Roe, A. W. Functionally specific optogenetic modulation in primate visual cortex. Proc. Natl Acad. Sci. USA 115, 10505–10510 (2018).
Yazdan-Shahmorad, A. et al. A large-scale interface for optogenetic stimulation and recording in nonhuman primates. Neuron 89, 927–939 (2016).
Chuong, A. S. et al. Noninvasive optical inhibition with a red-shifted microbial rhodopsin. Nat. Neurosci. 17, 1123–1129 (2014).
Yazdan-Shahmorad, A. et al. Demonstration of a setup for chronic optogenetic stimulation and recording across cortical areas in non-human primates. In Proceedings Volume 9305, Optical Techniques in Neurosurgery, Neurophotonics, and Optogenetics II (ed. Hirschberg, H.) 93052K (International Society for Optics and Photonics, 2015).
Komatsu, M., Sugano, E., Tomita, H. & Fujii, N. A chronically implantable bidirectional neural interface for non-human primates. Front. Neurosci. 11, 514 (2017).
Gong, X. et al. An ultra-sensitive step-function opsin for minimally invasive optogenetic stimulation in mice and macaques. Neuron 107, 38–51.e8 (2020).
Marshel, J. H. et al. Cortical layer–specific critical dynamics triggering perception. Science 365, 6453 (2019).
Owen, S. F., Liu, M. H. & Kreitzer, A. C. Thermal constraints on in vivo optogenetic manipulations. Nat. Neurosci. 22, 1061–1065 (2019).
Cox, D. D., Papanastassiou, A. M., Oreper, D., Andken, B. B. & Dicarlo, J. J. High-resolution three-dimensional microelectrode brain mapping using stereo microfocal X-ray imaging. J. Neurophysiol. 100, 2966–2976 (2008).
Issa, E. B., Papanastassiou, A. M. & DiCarlo, J. J. Large-scale, high-resolution neurophysiological maps underlying fMRI of macaque temporal lobe. J. Neurosci. 33, 15207–15219 (2013).
Fredericks, J. M. et al. Methods for mechanical delivery of viral vectors into rhesus monkey brain. J. Neurosci. Methods 339, 108730 (2020).
Salzman, C. D., Murasugi, C. M., Britten, K. H. & Newsome, W. T. Microstimulation in visual area MT: effects on direction discrimination performance. J. Neurosci. 12, 2331–2355 (1992).
Acknowledgements
We thank E. S. Boyden and J. G. Bernstein for their critical help during the early stages of array development. This research was supported by the Simons Foundation (SCGB (grant no. 325500) to J.J.D.), US National Eye Institute grant no. R01-EY014970 (to J.J.D.) and NIH grant no. K99 EY022924 (to A.A.), and by the Intramural Research Program of the NIMH grant no. ZIAMH002958 (to A.A.).
Author information
Authors and Affiliations
Contributions
A.A. developed the concept and general characteristics of the Opto-Array. M.S. designed and fabricated the Opto-Array, with guidance from A.A. and J.J.D. R.R. performed the Opto-Array photometric experiments. S.B. and R.A. performed the Opto-Array thermal experiments, with guidance from A.A. R.R. and A.A. performed the optical fiber experiments, with guidance from J.J.D. R.R. performed the Opto-Array behavioral experiments in V1 cortex in monkey Y, with guidance from A.A. and J.J.D. R.R. performed the Opto-Array neural experiments in IT cortex in monkey M, with guidance from A.A. and J.J.D. S.B. and R.A. performed the Opto-Array behavioral experiments in IT cortex in monkey S, with guidance from A.A. R.R. and A.A. wrote the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
M.S. is a principal engineer at Blackrock Microsystems (Utah). Blackrock will sell the Opto-Array and is bound to financially benefit from this work.
Additional information
Peer review information Nature Methods thanks Adriana Galvan, Atsushi Nambu and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Nina Vogt was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Thermal response.
(a) Photographs of the brain surface after explantation of Opto-Array after 155 days implanted in monkey Y. The photographs show the skull with craniotomy and durotomy over the right dorsal V1. The brain surface under each array is exposed and photographed. (b) Comparison of the light power output of a new Opto-Array to one that was implanted in an animal for 155 days (mean + - SD over repetitions, n = 80 repetitions). (c) Average thermal response from implanted Opto-Array to 36 different LED conditions, varying in power, duration, and number of illuminated LEDs. (d) Finite element model for simulating the temperature changes on the surface of the brain by solving the heat equation. For simplicity, we considered a 2D cross-section of the array on the brain surface and built a 2D surface consisting of a PCB contained within a silicone encapsulation, adjacent to brain tissue (top). (e) Temperature increases on the silicone encapsulation (blue) and the brain surface (red) after setting the boundary conditions of the PCB-to-silicone edge (yellow). (f) The simulated time course of temperature changes over the array and brain surface at 200 ms intervals.
Extended Data Fig. 2 Neural suppression using optrodes in V1 cortex.
We recorded V1 responses to a brief full-field grating stimulus, shown under a rapid serial visual presentation (RSVP) protocol, interleaving trials with and without light delivery from the acutely inserted optic fiber coupled to a green light laser. (a) Example rasters and peri-stimulus time histograms (PSTHs) from fiber optic experiments for 200 ms light duration (left) and 900 ms light duration (middle, right). Note the presence of a photoelectric artefact during light onset and offset in the left panel. Shaded lines correspond to mean + - SE, over n > 30 repetitions. (b) The percentage change in neural response between laser ON and control conditions, averaged over all recorded neural sites. Shaded lines correspond to mean + - SE, over n = 21 multi-unit sites (left) and n = 9 multi-unit sites (right). The green overlays correspond to the time of LED illumination. Over all recorded neural sites, the neural suppression by light delivery was statistically significant (p < 0.0001, one-sided exact test). Across all panels, asterisks are defined as: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Extended Data Fig. 3 Behavioral effects of optogenetic suppression in V1 cortex.
(a) Behavioral effects of optogenetic suppression of V1 cortex with light delivered via an acutely inserted fiber, for the luminance discrimination task in monkey Y. Formatting as in Fig. 2e,f. We observe substantial psychometric shifts in the region of interest within the visual field (mean + - SE over trials; n~10 trials per condition). (b) Optogenetic suppression of V1 cortex with light delivered via the Opto-Array, for the luminance discrimination task in monkey Y, yielded no substantial reaction time effects. The map shows the change in reaction time between control and illumination trials, pooling over all LED conditions. (c) Global effect from Opto-Array experiments, for all parameters of psychometric function, pooling over all LED conditions. We observe a reliable behavioral effect of LED illumination even at this coarse scale in the form of a substantial psychometric shift away from the ROI (α), but not with respect to any other psychometric parameters.
Extended Data Fig. 4 Neural suppression using Opto-Array in IT cortex.
(a) (top) Coronal MRI slice highlighting the location of the implanted Opto-Array on the lateral surface of area IT (inferior temporal cortex), with the location of the recording chamber on the dorsal surface (yellow) and acutely inserted electrode driven just beneath the array (red). (bottom) High-resolution micro-focal stereo x-ray system to precisely guide our electrodes close to the array surface. (b) Example stereo x-ray images showing two x-ray projections of the electrode, Opto-Array, and other skull implants (here, titanium straps and mesh used for Opto-Array implant). (c) 3D reconstruction of recorded IT sites relative to the Opto-Array. The red square shows the illuminated LED on the Opto-Array and the colored dots show all 31 corresponding recorded IT sites. Marker color corresponds to the distance between the recorded IT and the illuminated LED, on the plane parallel to the Opto-Array. (d) For the example IT site shown in c, the PSTH for control and illuminated conditions are shown in blue and red. Shaded lines correspond to mean + - SE, over n > 30 repetitions. (e) The percentage change in neural response between light ON and control conditions. Neural sites were split into two groups based on the 3D distance between the site location and the location of the illuminated LED (‘neighboring’ (left) and ‘distant’ (right) sites, defined as less than and greater than the median distance over all sites, respectively). Shaded lines correspond to mean + - SE, over n = 16 multi-unit sites (left) and n = 15 multi-unit sites (right). The green overlays correspond to the time of LED illumination. Over all recorded neighboring neural sites, the neural suppression by light delivery was statistically significant (p < 0.01, one-sided exact test). Distant IT sites did not show reliable suppression (p > 0.1, one-sided exact test). Across all panels, asterisks are defined as: *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
Extended Data Fig. 5 Behavioral deficits of optogenetic suppression in IT cortex.
(a) Behavioural paradigm: the animal was trained to detect optogenetic stimulation of its IT cortex. Each trial started by presenting a central fixation point and, after 500 ms of initial fixation, a visual stimulus (an image of a butterfly in this example), followed by two choice targets. This visual stimulus was presented in all conditions and was not predictive of the brain stimulation condition. (b) Time course of behavioral task. (c) We placed two arrays in similar cortical areas (IT cortex), one that had been transfected by virus, activated in the ‘stimulation trials’, and one that had not, activated in the ‘catch trials’ (10% of the trials). The three trial types were presented in random order. (d) The markers show the percentage of trials where the animal ‘reported stimulation’, as a function of training sessions separately for each trial type. The shaded areas represent 95% confidence intervals.
Supplementary information
Supplementary Information
Supplementary Tables 1–3.
Rights and permissions
About this article
Cite this article
Rajalingham, R., Sorenson, M., Azadi, R. et al. Chronically implantable LED arrays for behavioral optogenetics in primates. Nat Methods 18, 1112–1116 (2021). https://doi.org/10.1038/s41592-021-01238-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-021-01238-9
This article is cited by
-
Perceptography unveils the causal contribution of inferior temporal cortex to visual perception
Nature Communications (2024)
-
Body-conformable light-emitting materials and devices
Nature Photonics (2024)
-
An optrode array for spatiotemporally-precise large-scale optogenetic stimulation of deep cortical layers in non-human primates
Communications Biology (2024)
-
Wireless light energy harvesting and communication in a waterproof GaN optoelectronic system
Communications Engineering (2022)