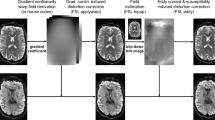
Abstract
Diffusion-weighted magnetic resonance imaging (dMRI) is the primary method for noninvasively studying the organization of white matter in the human brain. Here we introduce QSIPrep, an integrative software platform for the processing of diffusion images that is compatible with nearly all dMRI sampling schemes. Drawing on a diverse set of software suites to capitalize on their complementary strengths, QSIPrep facilitates the implementation of best practices for processing of diffusion images.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
Source data for Fig. 2 and Supplementary Tables 1–4 are available at https://pennlinc.github.io/qsiprep_paper/ (DOI 10.5281/zenodo.4667846). In addition, source data for Fig. 2 and Extended Data Fig. 2 are provided with this paper. Most imaging data are available from public repositories. PNC data (DTI64) are available on dbGAP (https://www.ncbi.nlm.nih.gov/projects/gap/cgi-bin/study.cgi?study_id=phs000607.v3.p2). ABCD data are publicly available in both raw BIDS (https://nda.nih.gov/edit_collection.html?id=3165) and preprocessed (https://nda.nih.gov/edit_collection.html?id=2573) states. HBN data are available on the NeuroImaging Tools and Resources Collaboratory (https://fcon_1000.projects.nitrc.org/indi/cmi_healthy_brain_network/sharing_neuro.html) under a data use agreement. Other imaging datasets did not obtain explicit participant consent for data to be placed in a public repository. HCP-Lifespan imaging data are available on request to G.K.A. DSI258 is available on request to J.M.V. DSI 756 is available on request to D.S.B. CS-DSI and MultiShell 113 are available on request to T.D.S. Source data are provided with this paper.
Code availability
All code used to perform the statistical tests are available at: https://pennlinc.github.io/qsiprep_paper/ (ref. 44) and the QSIPrep source code is available at github.com/pennbbl/qsiprep. Docker images for the versions of QSIPrep used in this paper are available on DockerHub at v.0.8.0 and 0.9.0beta1. Newer versions are available. A compute capsule containing QSIPrep and example data is available on Code Ocean45.
References
Wedeen, V. J., Hagmann, P., Tseng, W.-Y. I., Reese, T. G. & Weisskoff, R. M. Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn. Reson. Med. 54, 1377–1386 (2005).
Alexander, D. C. A general framework for experiment design in diffusion MRI and its application in measuring direct tissue-microstructure features. Magn. Reson. Med. 60, 439–448 (2008).
Fick, R. H. J., Wassermann, D., Caruyer, E. & Deriche, R. MAPL: tissue microstructure estimation using Laplacian-regularized MAP-MRI and its application to HCP data. Neuroimage 134, 365–385 (2016).
Yeh, C. H., Smith, R. E., Liang, X., Calamante, F. & Connelly, A. Correction for diffusion MRI fibre tracking biases: the consequences for structural connectomic metrics. Neuroimage 142, 150–162 (2016).
Gorgolewski, K. J. et al. The brain imaging data structure, a format for organizing and describing outputs of neuroimaging experiments. Sci. Data 3, 160044 (2016).
Andersson, J. L. R. & Sotiropoulos, S. N. An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125, 1063–1078 (2016).
Yeh, F.-C. & Tseng, W.-Y. I. NTU-90: a high angular resolution brain atlas constructed by q-space diffeomorphic reconstruction. Neuroimage 58, 91–99 (2011).
Garyfallidis, E. et al. Dipy, a library for the analysis of diffusion MRI data. Front. Neuroinform. 8, 8 (2014).
Avants, B. B., Epstein, C. L., Grossman, M. & Gee, J. C. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal. 12, 26–41 (2008).
Tournier, J. D. et al. MRtrix3: a fast, flexible and open software framework for medical image processing and visualisation. NeuroImage 202, 116137 (2019).
Gorgolewski, K. J. et al. BIDS apps: improving ease of use, accessibility, and reproducibility of neuroimaging data analysis methods. PLoS Comput. Biol. 13, e1005209 (2017).
Satterthwaite, T. D. et al. Neuroimaging of the Philadelphia Neurodevelopmental Cohort. NeuroImage 86, 544–553 (2014).
Hagler, D. J. et al. Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage 202, 116091 (2019).
Pines, A. R. et al. Leveraging multi-shell diffusion for studies of brain development in youth and young adulthood. Dev. Cogn. Neurosci. 43, 100788 (2020).
Harms, M. P. et al. Extending the Human Connectome Project across ages: imaging protocols for the Lifespan Development and Aging projects. Neuroimage 183, 972–984 (2018).
O’Connor, D. et al. The Healthy Brain Network Serial Scanning Initiative: a resource for evaluating inter-individual differences and their reliabilities across scan conditions and sessions. Gigascience 6, giw011 (2017).
Paquette, M., Merlet, S., Gilbert, G., Deriche, R. & Descoteaux, M. Comparison of sampling strategies and sparsifying transforms to improve compressed sensing diffusion spectrum imaging. Magn. Reson. Med. 73, 401–416 (2015).
Yeh, F. C. et al. Differential tractography as a track-based biomarker for neuronal injury. Neuroimage 202, 116131 (2019).
Veraart, J. et al. Denoising of diffusion MRI using random matrix theory. Neuroimage 142, 394–406 (2016).
Fadnavis, S., Batson, J. & Garyfallidis, E. Patch2Self: denoising diffusion MRI with self-supervised learning. in Proc. Advances in Neural Information Processing Systems Vol. 33 (eds Larochelle, H. et al.) 16293–16303 (Curran Associates, 2020).
Gorgolewski, K. et al. Nipype: a flexible, lightweight and extensible neuroimaging data processing framework in Python. Front. Neuroinform. 5, https://doi.org/10.3389/fninf.2011.00013 (2011).
Esteban, O. et al. fMRIPrep: a robust preprocessing pipeline for functional MRI. Nat. Methods 16, 111–116 (2019).
Veraart, J., Sijbers, J., Sunaert, S., Leemans, A. & Jeurissen, B. Weighted linear least squares estimation of diffusion MRI parameters: Strengths, limitations, and pitfalls. Neuroimage 81, 335–346 (2013).
Kellner, E., Dhital, B., Kiselev, V. G. & Reisert, M. Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn. Reson. Med. 76, 1574–1581 (2016).
Tustison, N. J. et al. N4ITK: improved N3 bias correction. IEEE Trans. Med. Imaging 29, 1310–1320 (2010).
Avants, B. B. et al. The optimal template effect in hippocampus studies of diseased populations. Neuroimage 49, 2457–2466 (2010).
Sotiropoulos, S. N. et al. Advances in diffusion MRI acquisition and processing in the Human Connectome Project. Neuroimage 80, 125–143 (2013).
Dhollander, T., Raffelt, D. & Connelly, A. Unsupervised 3-tissue response function estimation from single-shell or multi-shell diffusion MR data without a co-registered T1 image. in Proc. ISMRM Workshop on Breaking the Barriers of Diffusion MRI 5 (2016).
Smith, R. E., Tournier, J. D., Calamante, F. & Connelly, A. Anatomically-constrained tractography: improved diffusion MRI streamlines tractography through effective use of anatomical information. Neuroimage 62, 1924–1938 (2012).
Yeh, F.-C., Wedeen, V. J. & Tseng, W.-Y. I. Generalized q-sampling imaging. IEEE Trans. Med. Imaging 29, 1626–1635 (2010).
Yeh, F. C., Verstynen, T. D., Wang, Y., Fernández-Miranda, J. C. & Tseng, W. Y. I. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PLoS ONE 8, e80713 (2013).
Yeh, F. C., Wedeen, V. J. & Tseng, W. Y. I. Practical crossing fiber imaging with combined DTI datasets and generalized reconstruction algorithm. in Proc. Intl Soc. Magnetic Resonance Med. 365 (2009).
Özarslan, E. et al. Mean apparent propagator (MAP) MRI: a novel diffusion imaging method for mapping tissue microstructure. Neuroimage 78, 16–32 (2013).
Özarslan, E., Koay, C. G. & Basser, P. J. in Applied and Numerical Harmonic Analysis (eds Özarslan, E. et al.) 373–399 (Springer International Publishing, 2013).
Yeh, F.-C. & Verstynen, T. D. Converting multi-shell and diffusion spectrum imaging to high angular resolution diffusion imaging. Front. Neurosci. 10, 418 (2016).
Fan, L. et al. The Human Brainnetome Atlas: a new brain atlas based on connectional architecture. Cereb. Cortex 26, 3508–3526 (2016).
Joliot, M. et al. AICHA: an atlas of intrinsic connectivity of homotopic areas. J. Neurosci. Methods 254, 46–59 (2015).
Gordon, E. M. et al. Generation and evaluation of a cortical area parcellation from resting-state correlations. Cereb. Cortex 26, 288–303 (2016).
Tzourio-Mazoyer, N., Landeau, B. & Papathanassiou, D. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15, 273–289 (2002).
Power, J. D., Fair, D. A. & Schlaggar, B. L. Development of human functional brain networks. Neuron 67, 735–748 (2010).
Roalf, D. R. et al. The impact of quality assurance assessment on diffusion tensor imaging outcomes in a large-scale population-based cohort. Neuroimage 125, 903–919 (2016).
Zhang, H., Schneider, T., Wheeler-Kingshott, C. A. & Alexander, D. C. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. Neuroimage 61, 1000–1016 (2012).
Glasser, M. F. et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 80, 105–124 (2013).
Cieslak, M. PennLINC/qsiprep_paper: Publication Version. Zenodo https://doi.org/10.5281/zenodo.4667846 (2021)
Cieslak, M. et al. (2021) QSIPrep: An integrative platform for preprocessing and reconstructing diffusion MRI data [Source Code]. https://doi.org/10.24433/CO.6311778.v1 (2021).
Acknowledgements
Funding came from the following: grant nos. R01 MH111886 for D.J.O., D.S.B and T.D.S., NICHD R01HD09586101 to J.D.Y., NINDS R01-NS099348-01 for X.H. and D.S.B., UL1TR001878 for J.D. and M.B.K., 1 U01 EY025864-01 to G.K.A., P30 EY001583 to the Vision Research Center, MH080243 and Staunton Farm Foundation for B.S.L., T32 MH 018951 for L.M.C., R01MH113550, RF1MH116920, R01MH120482 for T.D.S., CBICA Software Seed grants for M.C. and A.A., no. R01-EB027585-01 for E.G., S.F., A.R., A.H.R. and A.K., W911NF-16-1-0474 from the Army Research Office and by the Institute for Collaborative Biotechnologies under Cooperative Agreement no. W911NF-19-2-0026 with the Army Research Office to S.T.G. and J.M.V., RF1AG054409 to C.D. B.S.L. was supported by grant no. T32MH014654. Support for the collection of the data for Philadelphia Neurodevelopment Cohort (PNC) was provided by grant no. RC2MH089983 awarded to R.E.G. Data used in the preparation of this article were obtained from the ABCD Study (https://abcdstudy.org), held in the NIMH Data Archive. This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9–10 years and follow them over 10 years into early adulthood. The ABCD Study is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123 and U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortium_members/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This paper reflects the views of the authors and may not reflect the opinions or views of the National Institutes of Health or ABCD consortium investigators.
Author information
Authors and Affiliations
Contributions
M.C., P.A.C., X.H., F.-C.Y., T.D., A.A., M.A.E., S.F., W.F., E.G., A.K., A.R.-H., J.B., L.M.C., P.F., T.M.T., A.P.M., V.J.S., U.A.T., J.D.Y., S.T.G. and T.D.S. developed QSIPrep by code contribution, testing and documentation. Data were collected, curated and shared by G.K.A., D.S.B., R.F.B., C.D., J.A.D., E.E., D.A.F., B.G., R.C.G., R.E.G., M.B.K., B.S.L., A.P.M., M.P.M., D.J.O., A.P., A.R., J.M.V. and S.T.G. Preprocessing pipelines (scheme-specific or QSIPrep) were run and shared by G.K.A., R.F.B., J.B., P.F., A.K., A.R.P., D.R.R., A.R.-H. and A.R. Statistical tests were designed and implemented by M.C., B.S.L., T.M.T. and T.D.S.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Methods thanks Jonathan D. Clayden and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Nina Vogt was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Diffusion imaging data used in QSIPrep development and evaluation.
Cartesian (DSI), random (CS-DSI), and shelled (single-shell DTI and multi-shell) sequences were used to test the preprocessing and reconstruction workflows in QSIPrep. Sequences varied widely in their maximum b-value (1000–5000 s/mm2), number of q-space samples (64–789) and voxel size (1.5–2.3 mm). The row colors represent these schemes across all figures. The colors in the HCP-Lifespan image indicate that these samples came from different scans, grouped by phase-encoding direction.
Extended Data Fig. 2 Comparing added smoothness from QSIPrep and previous pipelines.
Preprocessing generally increases the spatial smoothness of images relative to the raw images. Here the raw image smoothness (x-axis) is compared to the same images after being processed by the published pipeline for each dataset (left) and QSIPrep (right). The direct comparison between QSIPrep and the Previous Pipeline is presented in Fig. 2.
Extended Data Fig. 3 QSIPrep reconstruction workflows produce comparable output across diverse sampling schemes and reconstruction methods.
Four sampling schemes each reconstructed using four methods: GQI from DSI Studio, multi-tissue CSD from MRtrix, and MAPL from Dipy. ODF fields are shown in two white matter regions (left), a single fiber area in the corpus callosum (top) and a crossing fiber region in the centrum semiovale (bottom). The middle panel shows ODFs reconstructed in the single fiber region, and the right panel shows ODFs reconstructed in the crossing fiber region for the four sampling schemes (rows) and the three reconstruction methods (columns).
Supplementary information
Supplementary Information
Supplementary Figs. 1–3, Tables 1–5 and Notes 1–4.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Rights and permissions
About this article
Cite this article
Cieslak, M., Cook, P.A., He, X. et al. QSIPrep: an integrative platform for preprocessing and reconstructing diffusion MRI data. Nat Methods 18, 775–778 (2021). https://doi.org/10.1038/s41592-021-01185-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-021-01185-5
This article is cited by
-
Connectome-based modelling of neurodegenerative diseases: towards precision medicine and mechanistic insight
Nature Reviews Neuroscience (2023)
-
Myelination and excitation-inhibition balance synergistically shape structure-function coupling across the human cortex
Nature Communications (2023)
-
Intracranial electrophysiological and structural basis of BOLD functional connectivity in human brain white matter
Nature Communications (2023)
-
Towards a biologically annotated brain connectome
Nature Reviews Neuroscience (2023)
-
White Matter Characteristics of Damage Along Fiber Tracts in Patients with Type 2 Diabetes Mellitus
Clinical Neuroradiology (2023)