Abstract
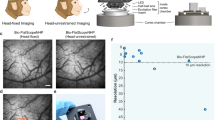
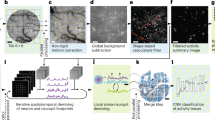
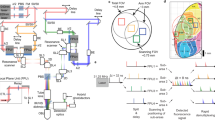
The advent of genetically encoded calcium indicators, along with surgical preparations such as thinned skulls or refractive-index-matched skulls, has enabled mesoscale cortical activity imaging in head-fixed mice. However, neural activity during unrestrained behavior substantially differs from neural activity in head-fixed animals. For whole-cortex imaging in freely behaving mice, we present the ‘mini-mScope’, a widefield, miniaturized, head-mounted fluorescence microscope that is compatible with transparent polymer skull preparations. With a field of view of 8 × 10 mm2 and weighing less than 4 g, the mini-mScope can image most of the mouse dorsal cortex with resolutions ranging from 39 to 56 µm. We used the mini-mScope to record mesoscale calcium activity across the dorsal cortex during sensory-evoked stimuli, open field behaviors, social interactions and transitions from wakefulness to sleep.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data containing videos, pseudocolor maps and images are available upon request from the authors due to the large file sizes. The Allen Brain Atlas was used as an anatomical reference for data analysis in this study (http://www.brain-map.org). All CAD files for manufacturing the mini-mScope are available with this article as Supplementary Data 2. Source data are provided with this paper.
Code availability
All custom code is available on Github at https://github.com/bsbrl/mini-mScope.
References
Chen, T.-W. et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 499, 295–300 (2013).
Daigle, T. L. et al. A suite of transgenic driver and reporter mouse lines with enhanced brain-cell-type targeting and functionality. Cell 174, 465–480.e22 (2018).
Vanni, M. P. & Murphy, T. H. Mesoscale transcranial spontaneous activity mapping in GCaMP3 transgenic mice reveals extensive reciprocal connections between areas of somatomotor cortex. J. Neurosci. 34, 15931–15946 (2014).
Allen, W. E. et al. Global representations of goal-directed behavior in distinct cell types of mouse neocortex. Neuron 94, 891–907.e6 (2017).
Wekselblatt, J. B., Flister, E. D., Piscopo, D. M. & Niell, C. M. Large-scale imaging of cortical dynamics during sensory perception and behavior. J. Neurophysiol. 115, 2852–2866 (2016).
Makino, H. et al. Transformation of cortex-wide emergent properties during motor learning. Neuron 94, 880–890.e8 (2017).
Musall, S., Kaufman, M. T., Juavinett, A. L., Gluf, S. & Churchland, A. K. Single-trial neural dynamics are dominated by richly varied movements. Nat. Neurosci. 22, 1677–1686 (2019).
Gilad, A. & Helmchen, F. Spatiotemporal refinement of signal flow through association cortex during learning. Nat. Commun. 11, 1744 (2020).
Mohajerani, M. H. et al. Spontaneous cortical activity alternates between motifs defined by regional axonal projections. Nat. Neurosci. 16, 1426–1435 (2013).
Ferezou, I. et al. Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron 56, 907–923 (2007).
Niu, Y. et al. Generation of gene-modified cynomolgus monkey via Cas9/RNA-mediated gene targeting in one-cell embryos. Cell 156, 836–843 (2014).
Pinto, L. et al. Task-dependent changes in the large-scale dynamics and necessity of cortical regions. Neuron 104, 810–824.e9 (2019).
Murphy, T. H. et al. High-throughput automated home-cage mesoscopic functional imaging of mouse cortex. Nat. Commun. 7, 11611 (2016).
Meyer, A. F., O’Keefe, J. & Poort, J. Two distinct types of eye-head coupling in freely moving mice. Curr. Biol. 30, 2116–2130.e6 (2020).
Juczewski, K., Koussa, J. A., Kesner, A. J., Lee, J. O. & Lovinger, D. M. Stress and behavioral correlates in the head-fixed method: stress measurements, habituation dynamics, locomotion, and motor-skill learning in mice. Sci. Rep. 10, 12245 (2020).
Aghajan, Z. M. et al. Impaired spatial selectivity and intact phase precession in two-dimensional virtual reality. Nat. Neurosci. 18, 121–128 (2015).
Ghosh, K. K. et al. Miniaturized integration of a fluorescence microscope. Nat. Methods 8, 871–878 (2011).
Zong, W. et al. Fast high-resolution miniature two-photon microscopy for brain imaging in freely behaving mice. Nat. Methods 14, 713–719 (2017).
Skocek, O. et al. High-speed volumetric imaging of neuronal activity in freely moving rodents. Nat. Methods 15, 429–432 (2018).
Scott, B. B. et al. Imaging cortical dynamics in GCaMP transgenic rats with a head-mounted widefield macroscope. Neuron 100, 1045–1058.e5 (2018).
Namiki, S., Sakamoto, H., Iinuma, S., Iino, M. & Hirose, K. Optical glutamate sensor for spatiotemporal analysis of synaptic transmission. Eur. J. Neurosci. 25, 2249–2259 (2007).
Marvin, J. S. et al. An optimized fluorescent probe for visualizing glutamate neurotransmission. Nat. Methods 10, 162–170 (2013).
Dana, H. et al. Thy1-GCaMP6 transgenic mice for neuronal population imaging in vivo. PLoS ONE 9, e108697 (2014).
Ghanbari, L. et al. Cortex-wide neural interfacing via transparent polymer skulls. Nat. Commun. 10, 1500 (2019).
Ma, Y. et al. Resting-state hemodynamics are spatiotemporally coupled to synchronized and symmetric neural activity in excitatory neurons. Proc. Natl Acad. Sci. USA 113, E8463–E8471 (2016).
Dubbs, A., Guevara, J. & Yuste, R. moco: fast motion correction for calcium imaging. Front. Neuroinform. 10, 6 (2016).
Dash, M. B., Douglas, C. L., Vyazovskiy, V. V., Cirelli, C. & Tononi, G. Long-term homeostasis of extracellular glutamate in the rat cerebral cortex across sleep and waking states. J. Neurosci. 29, 620–629 (2009).
Abadchi, J. K. et al. Spatiotemporal patterns of neocortical activity around hippocampal sharp-wave ripples. Elife 9, e51972 (2020).
Patti, C. L. et al. Effects of sleep deprivation on memory in mice: role of state-dependent learning. Sleep 33, 1669–1679 (2010).
Colavito, V. et al. Experimental sleep deprivation as a tool to test memory deficits in rodents. Front. Syst. Neurosci. 7, 106 (2013).
Mohajerani, M. H., McVea, D. A., Fingas, M. & Murphy, T. H. Mirrored bilateral slow-wave cortical activity within local circuits revealed by fast bihemispheric voltage-sensitive dye imaging in anesthetized and awake mice. J. Neurosci. 30, 3745–3751 (2010).
Li, P. et al. Measuring sharp waves and oscillatory population activity with the genetically encoded calcium indicator GCaMP6f. Front. Cell. Neurosci. 13, 274 (2019).
Dana, H. et al. High-performance calcium sensors for imaging activity in neuronal populations and microcompartments. Nat. Methods 16, 649–657 (2019).
Madisen, L. et al. Transgenic mice for intersectional targeting of neural sensors and effectors with high specificity and performance. Neuron 85, 942–958 (2015).
Dawson, T. M., Golde, T. E. & Lagier-Tourenne, C. Animal models of neurodegenerative diseases. Nat. Neurosci. 21, 1370–1379 (2018).
Cai, D. J. et al. A shared neural ensemble links distinct contextual memories encoded close in time. Nature 534, 115–118 (2016).
Juneau, J., Duret, G., Robinson, J. & Kemere, C. Enhanced image sensor module for head-mounted microscopes. In Proc. Annu. Int. Conf. IEEE Engineering in Medicine and Biology Society (EMBC) 826–829 (IEEE, 2018).
Barbera, G., Liang, B., Zhang, L., Li, Y. & Lin, D.-T. A wireless miniScope for deep brain imaging in freely moving mice. J. Neurosci. Methods 323, 56–60 (2019).
Valley, M. T. et al. Separation of hemodynamic signals from GCaMP fluorescence measured with wide-field imaging. J. Neurophysiol. 123, 356–366 (2020).
Lerner, T. N. et al. Intact-brain analyses reveal distinct information carried by SNc dopamine subcircuits. Cell 162, 635–647 (2015).
Dana, H. et al. Sensitive red protein calcium indicators for imaging neural activity. Elife 5, e12727 (2016).
Donaldson, P. D., Ghanbari, L., Rynes, M. L., Kodandaramaiah, S. B. & Swisher, S. L. Inkjet-printed silver electrode array for in-vivo electrocorticography. In Proc. Int. IEEE/EMBS Conf. Neural Engineering (NER) 774–777 (IEEE, 2019).
Ghanbari, L. et al. Craniobot: a computer numerical controlled robot for cranial microsurgeries. Sci. Rep. 9, 1023 (2019).
Rynes, M. L. et al. Assembly and operation of an open-source, computer numerical controlled (CNC) robot for performing cranial microsurgical procedures. Nat. Protoc. 15, 1992–2023 (2020).
Silasi, G., Xiao, D., Vanni, M. P., Chen, A. C. N. & Murphy, T. H. Intact skull chronic windows for mesoscopic wide-field imaging in awake mice. J. Neurosci. Methods 267, 141–149 (2016).
Pinheiro-da-Silva, J., Silva, P. F., Nogueira, M. B. & Luchiari, A. C. Sleep deprivation effects on object discrimination task in zebrafish (Danio rerio). Anim. Cogn. 20, 159–169 (2017).
Mathis, A. et al. DeepLabCut: markerless pose estimation of user-defined body parts with deep learning. Nat. Neurosci. 21, 1281–1289 (2018).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Lau, C. et al. Exploration and visualization of gene expression with neuroanatomy in the adult mouse brain. BMC Bioinformatics 9, 153 (2008).
Singh, S., Bermudez-Contreras, E., Nazari, M., Sutherland, R. J. & Mohajerani, M. H. Low-cost solution for rodent home-cage behaviour monitoring. PLoS ONE 14, e0220751 (2019).
Acknowledgements
S.B.K. acknowledges funds from the Mechanical Engineering Department, College of Science and Engineering, MnDRIVE RSAM initiative of the University of Minnesota, Minnesota Department of Higher Education and National Institutes of Health grant nos. 1R21NS103098-01, 1R01NS111028, 1R21NS112886, RF1NS113287 and 1R21NS111196. L.G. was supported by the University of Minnesota Informatics Institute graduate fellowship. D.A.S. was supported by the University of Minnesota Diversity of Views and Experiences fellowship. M.L.R. was supported by grant no. 1R21NS103098-01-01S1. M.H.M. acknowledges funding from Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant #40352.
Author information
Authors and Affiliations
Contributions
M.L.R., L.G., M.L., D.A.S., L.G., G.W.J. and S.B.K. designed and engineered the mini-mScope. M.L.R., D.A.S., J.D., Z.S.N., O.H., L.G. and S.B.K. designed and executed the experiments. M.L.R., D.A.S., S.L., O.H., V.R. and S.B.K. analyzed the data. M.L.R., D.A.S., S.L., V.R., J.D., M.L. and S.B.K. wrote the manuscript. M.N. and M.H.M. designed and executed the glutamate imaging experiments, analyzed the data and assisted with manuscript writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–8 and Notes 1–7.
Supplementary Data 1
Source data for supplementary figures.
Supplementary Video 1
Open field behavior of mouse bearing the mini-mScope. Video depicting a mouse bearing a mini-mScope behaving freely in an open field arena. The mouse is free and comfortable to move throughout the entire arena and rear against the arena walls. The mouse appears undeterred by the mini-mScope or its associated wiring.
Supplementary Video 2
Stability of mesoscale imaging using the mini-mScope during freely moving behavior. Video with top view of mouse moving around in the open field. Epochs of 5–10 s of different behaviors are shown (locomotion, grooming and rearing). Inset: Motion-corrected video of the corresponding cortical calcium dynamics as captured by the mini-mScope.
Supplementary Video 3
Mesoscale calcium dynamics during social behavior. Video with top view of two mice moving around in the open field. Epochs of 10–15 s of different behaviors are shown (no contact and contact). Inset: Motion-corrected video of the corresponding cortical calcium dynamics as captured by the mini-mScope.
Supplementary Data 2
ZIP file containing three main elements. The first is a folder containing the CAD design files. The second is a folder containing the PCB design files. The third is an Excel sheet (Supplementary_File_1.xlsx) containing a master parts list.
Source data
Source Data Fig. 1
Mini-mScope resolution and illumination testing data.
Source Data Fig. 2
Data used to compare the calcium dynamics imaged with the mini-mScope to a conventional widefield epifluorescence macroscope.
Source Data Fig. 3
Data for the sensory stimulus-evoked responses imaged by the mini-mScope.
Source Data Fig. 4
Data for the mesoscale imaging of the cortex during free behavior taken with the mini-mScope.
Source Data Fig. 5
Data for the combined electrophysiological recording and mesoscale imaging of brain activity during sleep recorded with the mini-mScope.
Rights and permissions
About this article
Cite this article
Rynes, M.L., Surinach, D.A., Linn, S. et al. Miniaturized head-mounted microscope for whole-cortex mesoscale imaging in freely behaving mice. Nat Methods 18, 417–425 (2021). https://doi.org/10.1038/s41592-021-01104-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41592-021-01104-8
This article is cited by
-
Mesoscopic calcium imaging in a head-unrestrained male non-human primate using a lensless microscope
Nature Communications (2024)
-
Trans-segmental imaging in the spinal cord of behaving mice
Nature Biotechnology (2023)
-
An optical design enabling lightweight and large field-of-view head-mounted microscopes
Nature Methods (2023)
-
An optimized bioluminescent substrate for non-invasive imaging in the brain
Nature Chemical Biology (2023)
-
Large depth-of-field ultra-compact microscope by progressive optimization and deep learning
Nature Communications (2023)