Abstract
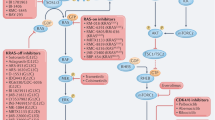
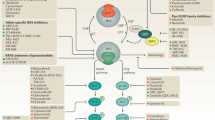
RAS family variants—most of which involve KRAS—are the most commonly occurring hotspot mutations in human cancers and are associated with a poor prognosis. For almost four decades, KRAS has been considered undruggable, in part due to its structure, which lacks small-molecule binding sites. But recent developments in bioengineering, organic chemistry and related fields have provided the infrastructure to make direct KRAS targeting possible. The first successes occurred with allele-specific targeting of KRAS p.Gly12Cys (G12C) in non-small cell lung cancer, resulting in regulatory approval of two agents—sotorasib and adagrasib. Inhibitors targeting other variants beyond G12C have shown preliminary antitumor activity in highly refractory malignancies such as pancreatic cancer. Herein, we outline RAS pathobiology with a focus on KRAS, illustrate therapeutic approaches across a variety of malignancies, including emphasis on the ‘on’ and ‘off’ switch allele-specific and ‘pan’ RAS inhibitors, and review immunotherapeutic and other key combination RAS targeting strategies. We summarize mechanistic understanding of de novo and acquired resistance, review combination approaches, emerging technologies and drug development paradigms and outline a blueprint for the future of KRAS therapeutics with anticipated profound clinical impact.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Karnoub, A. E. & Weinberg, R. A. Ras oncogenes: split personalities. Nat. Rev. Mol. Cell Biol. 9, 517–531 (2008).
Simanshu, D. K., Nissley, D. V. & McCormick, F. RAS proteins and their regulators in human disease. Cell 170, 17–33 (2017).
Ryan, M. B. & Corcoran, R. B. Therapeutic strategies to target RAS-mutant cancers. Nat. Rev. Clin. Oncol. 15, 709–720 (2018).
Hunter, J. C. et al. Biochemical and structural analysis of common cancer-associated KRAS mutations. Mol. Cancer Res. 13, 1325–1335 (2015).
Ostrem, J. M. et al. K-Ras(G12C) inhibitors allosterically control GTP affinity and effector interactions. Nature 503, 548–551 (2013).
Patricelli, M. P. et al. Selective inhibition of oncogenic KRAS output with small molecules targeting the inactive state. Cancer Discov. 6, 316–329 (2016).
Muller, M. P. et al. Nucleotide based covalent inhibitors of KRas can only be efficient in vivo if they bind reversibly with GTP-like affinity. Sci. Rep. 7, 3687 (2017).
Lito, P. et al. Allele-specific inhibitors inactivate mutant KRAS G12C by a trapping mechanism. Science 351, 604–608 (2016).
Zhao, Y., Xue, J. Y. & Lito, P. Suppressing nucleotide exchange to inhibit KRAS-mutant tumors. Cancer Discov. 11, 17–19 (2021).
Hallin, J. et al. The KRASG12C Inhibitor MRTX849 provides insight toward therapeutic susceptibility of KRAS-mutant cancers in mouse models and patients. Cancer Discov. 10, 54–71 (2020).
Canon, J. et al. The clinical KRASG12C inhibitor AMG 510 drives anti-tumour immunity. Nature 575, 217–223 (2019).
Salem, M. E. et al. Landscape of KRASG12C, associated genomic alterations, and interrelation with immuno-oncology biomarkers in KRAS-mutated cancers. JCO Precis. Oncol. 6, e2100245 (2022).
Prior, I. A., Hood, F. E. & Hartley, J. L. The frequency of Ras mutations in cancer. Cancer Res. 80, 2969–2974 (2020).
Consortium, A. P. G. AACR Project GENIE: powering precision medicine through an international consortium. Cancer Discov. 7, 818–831 (2017).
Cerami, E. et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2, 401–404 (2012).
Hong, D. S. et al. KRAS(G12C) inhibition with sotorasib in advanced solid tumors. N. Engl. J. Med. 383, 1207–1217 (2020).
Skoulidis, F. et al. STK11/LKB1 mutations and PD-1 inhibitor resistance in KRAS-mutant lung adenocarcinoma. Cancer Discov. 8, 822–835 (2018).
Janne, P. A. et al. Adagrasib in non-small-cell lung cancer harboring a KRASG12C mutation. N. Engl. J. Med. 387, 120–131 (2022).
Skoulidis, F. et al. Sotorasib for lung cancers with KRAS p.G12C mutation. N. Engl. J. Med. 384, 2371–2381 (2021).
de Langen, A. J. et al. Sotorasib versus docetaxel for previously treated non-small-cell lung cancer with KRASG12C mutation: a randomised, open-label, phase 3 trial. Lancet 401, 733–746 (2023).
Food and Drug Administration. Oncologic Drugs Advisory Committee Meeting, 5 October 2023. https://www.fda.gov/media/172696/
Sacher, A. et al. Single-agent divarasib (GDC-6036) in solid tumors with a KRAS G12C mutation. N. Engl. J. Med. 389, 710–721 (2023).
Murciano-Goroff, Y. R. et al. Abstract CT028: a first-in-human phase 1 study of LY3537982, a highly selective and potent KRAS G12C inhibitor in patients with KRAS G12C-mutant advanced solid tumors. Cancer Res. 83, CT028 (2023).
Cassier, P. A. et al. KontRASt-01 update: safety and efficacy of JDQ443 in KRAS G12C-mutated solid tumors including non-small cell lung cancer (NSCLC). J. Clin. Oncol. 41, 9007 (2023).
Li, Z. et al. D-1553 (Garsorasib), a potent and selective inhibitor of KRASG12C in patients with NSCLC: phase 1 study results. J. Thorac. Oncol. 18, 940–951 (2023).
Yuan, Y. et al. 106P efficacy and safety of IBI351 (GFH925) monotherapy in metastatic colorectal cancer harboring KRASG12C mutation: updated results from a pooled analysis of two phase I studies. Ann. Oncol. 34, S1512 (2023).
Zhou, Q. et al. LBA12 efficacy and safety of IBI351 (GFH925), a selective KRASG12C inhibitor, monotherapy in patients (pts) with advanced non-small cell lung cancer (NSCLC): Initial results from a registrational phase II study. Ann. Oncol. 34, S1662 (2023).
Negrao, M. V. et al. Intracranial efficacy of adagrasib in patients From the KRYSTAL-1 trial with KRASG12C-mutated non-small-cell lung cancer who have untreated CNS metastases. J. Clin. Oncol. 41, 4472–4477 (2023).
Ramalingam, S. et al. P52.03 efficacy of sotorasib in KRAS p.G12C-mutated NSCLC with stable brain metastases: a post-hoc analysis of CodeBreaK 100. J. Thorac. Oncol. 16, S1123 (2021).
Amodio, V. et al. EGFR blockade reverts resistance to KRASG12C inhibition in colorectal cancer. Cancer Discov. 10, 1129–1139 (2020).
Yaeger, R. Combination therapy and appropriate dosing to target KRAS in colorectal cancer. N. Engl. J. Med. 389, 2197–2199 (2023).
Corcoran, R. B. et al. EGFR-mediated re-activation of MAPK signaling contributes to insensitivity of BRAF mutant colorectal cancers to RAF inhibition with vemurafenib. Cancer Discov. 2, 227–235 (2012).
Fakih, M. G. et al. Sotorasib for previously treated colorectal cancers with KRASG12C mutation (CodeBreaK 100): a prespecified analysis of a single-arm, phase 2 trial. Lancet Oncol. 23, 115–124 (2022).
Yaeger, R. et al. Adagrasib with or without cetuximab in colorectal cancer with mutated KRAS G12C. N. Engl. J. Med. 388, 44–54 (2023).
Lito, P., Rosen, N. & Solit, D. B. Tumor adaptation and resistance to RAF inhibitors. Nat. Med. 19, 1401–1409 (2013).
Kopetz, S. et al. Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J. Clin. Oncol. 33, 4032–4038 (2015).
Prahallad, A. et al. Unresponsiveness of colon cancer to BRAFV600E inhibition through feedback activation of EGFR. Nature 483, 100–103 (2012).
Fakih, M. G. et al. Sotorasib plus panitumumab in refractory colorectal cancer with mutated KRAS G12C. N. Engl. J. Med. 389, 2125–2139 (2023).
Desai, J. et al. Divarasib plus cetuximab in KRAS G12C-positive colorectal cancer: a phase 1b trial. Nat Med. 30, 271–278 (2023).
Strickler, J. H. et al. Sotorasib in KRAS p.G12C-mutated advanced pancreatic cancer. N. Engl. J. Med. 388, 33–43 (2023).
Bekaii-Saab, T. S. et al. Adagrasib in advanced solid tumors harboring a KRASG12C mutation. J. Clin. Oncol. 41, 4097–4106 (2023).
Luo, J. KRAS mutation in pancreatic cancer. Semin. Oncol. 48, 10–18 (2021).
Kondo, S. et al. 1622P D-1553 in patients with KRAS G12C mutated advanced pancreatic cancer (PCa). Ann. Oncol. 34, S898–S899 (2023).
Hollebecque, A. et al. Efficacy and safety of LY3537982, a potent and highly selective KRAS G12C inhibitor in KRAS G12C-mutant GI cancers: results from a phase 1 study. J. Clin. Oncol. 42, 94 (2024).
Li, J. et al. Preliminary activity and safety results of KRAS G12C inhibitor glecirasib (JAB-21822) in patients with pancreatic cancer and other solid tumors. J. Clin. Oncol. 42, 604 (2024).
Subbiah, V. et al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J. Clin. Oncol. 36, 7–13 (2018).
Robert, C. et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N. Engl. J. Med. 372, 30–39 (2015).
Gershenson, D. M. et al. Trametinib versus standard of care in patients with recurrent low-grade serous ovarian cancer (GOG 281/LOGS): an international, randomised, open-label, multicentre, phase 2/3 trial. Lancet 399, 541–553 (2022).
Zhao, Y. et al. Diverse alterations associated with resistance to KRAS(G12C) inhibition. Nature 599, 679–683 (2021).
Awad, M. M. et al. Acquired resistance to KRASG12C inhibition in cancer. N. Engl. J. Med. 384, 2382–2393 (2021).
Tanaka, N. et al. Clinical acquired resistance to KRASG12C inhibition through a novel KRAS Switch-II pocket mutation and polyclonal alterations converging on RAS-MAPK reactivation. Cancer Discov. 11, 1913–1922 (2021).
Yaeger, R. et al. Molecular characterization of acquired resistance to KRASG12C-EGFR inhibition in colorectal cancer. Cancer Discov. 13, 41–55 (2023).
Dy, G. K. et al. Long-term outcomes and molecular correlates of sotorasib efficacy in patients with pretreated KRAS G12C-mutated non-small-cell lung cancer: 2-year analysis of CodeBreaK 100. J. Clin. Oncol. 41, 3311–3317 (2023).
Negrao, M. V. et al. Comutations and KRASG12C inhibitor efficacy in advanced NSCLC. Cancer Discov. 13, 1556–1571 (2023).
Thummalapalli, R. et al. Clinical and genomic features of response and toxicity to sotorasib in a real-world cohort of patients with advanced KRAS G12C-mutant non-small cell lung cancer. JCO Precis. Oncol. 7, e2300030 (2023).
Hallin, J. et al. Anti-tumor efficacy of a potent and selective non-covalent KRASG12D inhibitor. Nat. Med. 28, 2171–2182 (2022).
Gulay, K. C. M. et al. Dual Inhibition of KRASG12D and pan-ERBB is synergistic in pancreatic ductal adenocarcinoma. Cancer Res. 83, 3001–3012 (2023).
Ryan, M. B. et al. KRASG12C-independent feedback activation of wild-type RAS constrains KRASG12C inhibitor efficacy. Cell Rep. 39, 110993 (2022).
Li, Z., et al., Alveolar differentiation drives resistance to KRAS inhibition in lung adenocarcinoma. Cancer Discov. 14, 308–325 (2023).
Tong, X. et al. Adeno-to-squamous transition drives resistance to KRAS inhibition in LKB1 mutant lung cancer. Cancer Cell https://doi.org/10.1016/j.ccell.2024.01.012 (2024).
Schoenfeld, A. J. et al. Tumor analyses reveal squamous transformation and off-target alterations as early resistance mechanisms to first-line osimertinib in EGFR-mutant lung cancer. Clin. Cancer Res. 26, 2654–2663 (2020).
Singh, A. et al. A gene expression signature associated with ‘K-Ras addiction’ reveals regulators of EMT and tumor cell survival. Cancer Cell 15, 489–500 (2009).
Adachi, Y. et al. Epithelial-to-mesenchymal transition is a cause of both intrinsic and acquired resistance to KRAS G12C inhibitor in KRAS G12C-mutant non-small cell lung cancer. Clin. Cancer Res. 26, 5962–5973 (2020).
Tsai, Y. S. et al. Rapid idiosyncratic mechanisms of clinical resistance to KRAS G12C inhibition. J. Clin. Invest. 132, e155523 (2022).
Wang, X. et al. Identification of MRTX1133, a noncovalent, potent, and selective KRASG12D inhibitor. J. Med. Chem. 65, 3123–3133 (2022).
Zhou, C. et al. LBA33 A first-in-human phase I study of a novel KRAS G12D inhibitor HRS-4642 in patients with advanced solid tumors harboring KRAS G12D mutation. Ann. Oncol. 34, S1273 (2023).
Kemp, S. B. et al. Efficacy of a small-molecule inhibitor of KrasG12D in immunocompetent models of pancreatic cancer. Cancer Discov. 13, 298–311 (2023).
Mahadevan, K. K. et al. KRASG12D inhibition reprograms the microenvironment of early and advanced pancreatic cancer to promote FAS-mediated killing by CD8+ T cells. Cancer Cell 41, 1606–1620 (2023).
Schulze, C. J. et al. Chemical remodeling of a cellular chaperone to target the active state of mutant KRAS. Science 381, 794–799 (2023).
Jänne P. A. et al. Preliminary safety and anti-tumor activity of RMC-6291, a first-in-class, tri-complex KRAS G12C(ON) inhibitor in patients with or without prior KRAS G12C(OFF) inhibitor treatment. AACR-NCI-EORTC International Conference on Molecular Targets and Cancer Therapeutics, 11–15 October 2023.
Patel, S. et al. Abstract 1142: discovery of FMC-376 a novel orally bioavailable inhibitor of activated KRASG12C. Cancer Res. 83, 1142 (2023).
Lu, J. et al. D3S-001, a highly potent, selective, and differentiated covalent inhibitor of KRAS G12C: Human dose prediction and first‐in‐human (FIH) trial design. J. Clin. Oncol. 40, e15087 (2022).
Nagashima, T. et al. ASP3082, a First-in-class novel KRAS G12D degrader, exhibits remarkable anti-tumor activity in KRAS G12D mutated cancer models. Eur. J. Cancer 174, S30 (2022).
Singh, M., et al. Concurrent inhibition of oncogenic and wild-type RAS-GTP for cancer therapy. Preprint at Research Square https://doi.org/10.21203/rs.3.rs-3122478/v1 (2023).
Urszula, N. W., et al. Tumor-selective effects of active RAS inhibition in pancreatic ductal adenocarcinoma. Preprint at bioRxiv https://doi.org/10.1101/2023.12.03.569791 (2023).
Arbour, K. C. et al. 652O Preliminary clinical activity of RMC-6236, a first-in-class, RAS-selective, tri-complex RAS-MULTI(ON) inhibitor in patients with KRAS mutant pancreatic ductal adenocarcinoma (PDAC) and non-small cell lung cancer (NSCLC). Ann. Oncol. 34, S458 (2023).
Kim, D. et al. Pan-KRAS inhibitor disables oncogenic signalling and tumour growth. Nature 619, 160–166 (2023).
Corcoran, R. B. A single inhibitor for all KRAS mutations. Nat. Cancer 4, 1060–1062 (2023).
Tedeschi, A. et al. Abstract A085: BI KRASmulti, a first-in-class, orally bioavailable and direct inhibitor of diverse oncogenic KRAS variants drives tumor regression in preclinical models and validates wild-type amplified KRAS as a therapeutic target. Mol. Cancer Ther. 22, A085 (2023).
Johannes, P. et al. Targeting cancer with small molecule pan-KRAS degraders. Preprint at BioRxiv https://doi.org/10.1101/2023.10.24.563163 (2023).
Li, J., et al. Glecarisib (JAB-21822) monotherapy and in combination with cetuximab in patients with advanced colorectal cancer. in JCA-AACR Precision Cancer Medicine International Conference (2023).
Han, J. et al. Stromal-derived NRG1 enables oncogenic KRAS bypass in pancreas cancer. Genes Dev. 37, 818–828 (2023).
Gandara, D. et al. Abstract P05-02: a phase 1b study evaluating the combination of sotorasib, a KRASG12C inhibitor, and afatinib, a pan-ErbB tyrosine kinase inhibitor, in advanced KRAS p.G12C mutated non-small cell lung cancer (NSCLC). Mol. Cancer Ther. 20, P05-02 (2021).
Liu, C. et al. Combinations with allosteric SHP2 inhibitor TNO155 to block receptor tyrosine kinase signaling. Clin. Cancer Res. 27, 342–354 (2021).
Fedele, C. et al. SHP2 inhibition diminishes KRASG12C cycling and promotes tumor microenvironment remodeling. J. Exp. Med. 218, e20201414 (2021).
Nichols, R. J. et al. RAS nucleotide cycling underlies the SHP2 phosphatase dependence of mutant BRAF-, NF1- and RAS-driven cancers. Nat. Cell Biol. 20, 1064–1073 (2018).
Hofmann, M. H. et al. BI-3406, a potent and selective SOS1-KRAS interaction inhibitor, is effective in KRAS-driven cancers through combined MEK inhibition. Cancer Discov. 11, 142–157 (2021).
Brana, I. et al. Initial results from a dose finding study of TNO155, a SHP2 inhibitor, in adults with advanced solid tumors. J. Clin. Oncol. 39, 3005 (2021).
Ou, S. I. et al. A12 The SHP2 Inhibitor RMC-4630 in patients with KRAS-mutant non-small cell lung cancer: preliminary evaluation of a first-in-man phase 1 clinical trial. J. Thorac. Oncol. 15, S15–S16 (2020).
Koczywas, M. et al. Abstract LB001: anti-tumor activity and tolerability of the SHP2 inhibitor RMC-4630 as a single agent in patients with RAS-addicted solid cancers. Cancer Res. 81, LB001 (2021).
Drilon, A. et al. SHP2 inhibition sensitizes diverse oncogene-addicted solid tumors to re-treatment with targeted therapy. Cancer Discov. 13, 1789–1801 (2023).
Wang, J. et al. 653O Glecirasib (KRAS G12C inhibitor) in combination with JAB-3312 (SHP2 inhibitor) in patients with KRAS p.G12C mutated solid tumors. Ann. Oncol. 34, S459 (2023).
Negrão, M. et al. MA06.03 KontRASt-01: preliminary safety and efficacy of JDQ443 + TNO155 in patients with advanced, KRAS G12C-mutated solid tumors. J. Thorac. Oncol. 18, S117–S118 (2023).
Falchook, G. et al. OA03.03 Sotorasib in combination with RMC-4630, a SHP2 inhibitor, in KRAS p.G12C-mutated NSCLC and other solid tumors. J. Thorac. Oncol. 17, S8 (2022).
Ketcham, J. M. et al. Design and discovery of MRTX0902, a potent, selective, brain-penetrant, and orally bioavailable inhibitor of the SOS1:KRAS protein–protein interaction. J. Med. Chem. 65, 9678–9690 (2022).
Johnson, M. L. et al. 524P A phase I, open-label, dose-escalation trial of BI 1701963 in patients (pts) with KRAS mutated solid tumours: A snapshot analysis. Ann. Oncol. 32, S591–S592 (2021).
Misale, S. et al. KRAS G12C NSCLC models are sensitive to direct targeting of KRAS in combination with PI3K inhibition. Clin. Cancer Res. 25, 796–807 (2019).
Ramalingam, S. et al. Abstract P05-01: A phase 1b study evaluating the safety and efficacy of sotorasib, a KRASG12C inhibitor, in combination with trametinib, a MEK inhibitor, in KRAS p.G12C-mutated solid tumors. Mol. Cancer Ther. 20, P05-01 (2021).
Lito, P. et al. Disruption of CRAF-mediated MEK activation is required for effective MEK inhibition in KRAS mutant tumors. Cancer Cell 25, 697–710 (2014).
Mukhopadhyay, S. et al. Genome-wide CRISPR screens identify multiple synthetic lethal targets that enhance KRASG12C inhibitor efficacy. Cancer Res. 83, 4095–4111 (2023).
Edwards, A. C. et al. TEAD inhibition overcomes YAP1/TAZ-driven primary and acquired resistance to KRASG12C inhibitors. Cancer Res. 83, 4112–4129 (2023).
Prahallad, A. et al. CRISPR screening identifies mechanisms of resistance to KRASG12C and SHP2 inhibitor combinations in non-small cell lung cancer. Cancer Res. 83, 4130–4141 (2023).
Adachi, Y. et al. Scribble mis-localization induces adaptive resistance to KRAS G12C inhibitors through feedback activation of MAPK signaling mediated by YAP-induced MRAS. Nat. Cancer 4, 829–843 (2023).
Yap, T. A. et al. Abstract CT006: First-in-class, first-in-human phase 1 trial of VT3989, an inhibitor of yes-associated protein (YAP)/transcriptional enhancer activator domain (TEAD), in patients (pts) with advanced solid tumors enriched for malignant mesothelioma and other tumors with neurofibromatosis 2 (NF2) mutations. Cancer Res. 83, CT006 (2023).
Goodwin, C. M. et al. Combination therapies with CDK4/6 inhibitors to treat KRAS-mutant pancreatic cancer. Cancer Res. 83, 141–157 (2023).
Ruscetti, M. et al. NK cell-mediated cytotoxicity contributes to tumor control by a cytostatic drug combination. Science 362, 1416–1422 (2018).
Kinsey, C. G. et al. Protective autophagy elicited by RAF→MEK→ERK inhibition suggests a treatment strategy for RAS-driven cancers. Nat. Med. 25, 620–627 (2019).
Bryant, K. L. et al. Combination of ERK and autophagy inhibition as a treatment approach for pancreatic cancer. Nat. Med. 25, 628–640 (2019).
Lv, X. et al. Modulation of the proteostasis network promotes tumor resistance to oncogenic KRAS inhibitors. Science 381, eabn4180 (2023).
Liao, W. et al. KRAS-IRF2 axis drives immune suppression and immune therapy resistance in colorectal cancer. Cancer Cell 35, 559–572 (2019).
Pylayeva-Gupta, Y. et al. Oncogenic Kras-induced GM-CSF production promotes the development of pancreatic neoplasia. Cancer Cell 21, 836–847 (2012).
Coelho, M. A. et al. Oncogenic RAS signaling promotes tumor immunoresistance by stabilizing PD-L1 mRNA. Immunity 47, 1083–1099 (2017).
Chour, A. et al. Brief Report: severe sotorasib-related hepatotoxicity and non-liver adverse events associated with sequential anti-programmed cell death (ligand)1 and sotorasib therapy in KRAS(G12C)-mutant lung cancer. J. Thorac. Oncol. 18, 1408–1415 (2023).
Schoenfeld, A. J. et al. Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib. Ann. Oncol. 30, 839–844 (2019).
Li, B. T. et al. OA03.06 CodeBreaK 100/101: first report of safety/efficacy of sotorasib in combination with pembrolizumab or atezolizumab in advanced KRAS p.G12C NSCLC. J. Thorac. Oncol. 17, S10–S11 (2022).
Garassino, M. C. et al. LBA65 KRYSTAL-7: efficacy and safety of adagrasib with pembrolizumab in patients with treatment-naive, advanced non-small cell lung cancer (NSCLC) harboring a KRASG12C mutation. Ann. Oncol. 34, S1309–S1310 (2023).
Clarke, J. M. et al. MA06.05 CodeBreaK 101: safety and efficacy of sotorasib with carboplatin and pemetrexed in KRAS G12C-mutated advanced NSCLC. J. Thorac. Oncol. 18, S118–S119 (2023).
Sakata, S. et al. The primary endpoint analysis of SCARLET study: a single-arm, phase II study of sotorasib plus carboplatin-pemetrexed in patients with advanced non-squamous, non-small cell lung cancer with KRAS G12C mutation (WJOG14821L). J. Clin. Oncol. 41, 9006 (2023).
Masuishi, T. et al. 92MO Sotorasib (Soto) + panitumumab (Pmab) and FOLFIRI in previously treated KRASG12C-mutated metastatic colorectal cancer (mCRC): Safety and efficacy for CodeBreaK 101 phase Ib expansion cohort. Ann. Oncol. 34, S1505 (2023).
Abou-Alfa, G. K. et al. Targeting mutated K-ras in pancreatic adenocarcinoma using an adjuvant vaccine. Am. J. Clin. Oncol. 34, 321–325 (2011).
Weden, S. et al. Long-term follow-up of patients with resected pancreatic cancer following vaccination against mutant K-ras. Int. J. Cancer 128, 1120–1128 (2011).
Leidner, R. et al. Neoantigen T-cell receptor gene therapy in pancreatic cancer. N. Engl. J. Med. 386, 2112–2119 (2022).
Tran, E. et al. T-cell transfer therapy targeting mutant KRAS in cancer. N. Engl. J. Med. 375, 2255–2262 (2016).
Pant, S. et al. Lymph-node-targeted, mKRAS-specific amphiphile vaccine in pancreatic and colorectal cancer: the phase 1 AMPLIFY-201 trial. Nat. Med. 30, 531–542 (2024).
Huff, A. L. et al. Abstract LB197: a pooled mutant KRAS peptide vaccine activates polyfunctional T cell responses in patients with resected pancreatic cancer. Cancer Res. 83, LB197 (2023).
Olivier, T., Haslam, A. & Prasad, V. Sotorasib in KRASG12C mutated lung cancer: can we rule out cracking KRAS led to worse overall survival? Transl. Oncol. 28, 101591 (2023).
Paweletz, C. P. et al. Early changes in circulating cell-free KRAS G12C predict response to adagrasib in KRAS mutant non-small cell lung cancer patients. Clin. Cancer Res. 29, 3074–3080 (2023).
Gouda, M. A. et al. Liquid Biopsy Response Evaluation Criteria in Solid Tumors (LB-RECIST). Ann. Oncol. 35, 267–275 (2023).
Murciano-Goroff, Y. et al. Abstract 1144: dynamic changes in circulating tumor DNA (ctDNA) in patients treated with sotorasib for KRAS G12C mutant non-small cell lung cancer. Cancer Res. 83, 1144 (2023).
Ruan, D. -Y. et al. Safety and efficacy of D-1553 in KRAS G12C-mutated colorectal cancer: Results from a phase I/II study. J. Clin. Oncol. 41, 3563 (2023).
Zafra, M. P. et al. An in vivo kras allelic series reveals distinct phenotypes of common oncogenic variants. Cancer Discov. 10, 1654–1671 (2020).
Dogan, S. et al. Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin. Cancer Res. 18, 6169–6177 (2012).
Acknowledgements
Cancer Center Support Grant/Core Grant P30 CA008748. NCI/NIH P50 CA257881-01A1. NCI/NIH K12 CA184746.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
E.O.R. has received institutional research funding from Genentech/Roche, BioNTech, AstraZeneca, Arcus, Elicio, Parker Institute, NIH/NCI, Digestive Care, Revolution Medicine. Consulting/DSMB: Arcus, Alligator, Agenus, BioNTech, Ipsen, Merck, Moma Therapeutics, Novartis, Syros, Leap Therapeutics, Astellas, BMS, Fibrogen, Revolution Medicine, Merus, Agios (spouse), Genentech-Roche (spouse), Eisai (spouse) and Servier (Spouse). B.T.L. serves as an uncompensated advisor and consultant to Amgen, AstraZeneca, Boehringer Ingelheim, Bolt Biotherapeutics, Daiichi Sankyo, Genentech and Lilly. B.T.L. has received research grants to his institution from Amgen, AstraZeneca, Bolt Biotherapeutics, Daiichi Sankyo, Genentech, Jiangsu Hengrui Pharmaceuticals, Revolution Medicine, and Lilly. B.T.L. received academic travel support from Amgen. B.T.L. is an inventor on two institutional patents at MSK (US62/685,057, US62/514,661) and has intellectual property rights as a book author at Karger Publishers and Shanghai Jiao Tong University Press. A.S. declares no competing interests.
Peer review
Peer review information
Nature Medicine thanks Grainne O’Kane, Jayesh Desai, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Karen O’Leary, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Table 1
Frequency of acquired resistance mutations in MAPK pathway genes in recent clinical datasets.
Supplementary Table 2
Trials of combination therapy with KRAS inhibitors.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Singhal, A., Li, B.T. & O’Reilly, E.M. Targeting KRAS in cancer. Nat Med 30, 969–983 (2024). https://doi.org/10.1038/s41591-024-02903-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-024-02903-0