Abstract
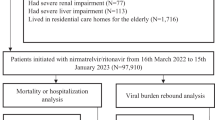
To date, there is a lack of randomized trial data examining the use of the antiviral nirmatrelvir/ritonavir in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-infected pregnant persons. This target trial emulation study aimed to address this gap by evaluating the use of nirmatrelvir/ritonavir in nonhospitalized pregnant women with symptomatic SARS-CoV-2 Omicron variant infection. Among patients diagnosed between 16 March 2022 and 5 February 2023, exposure was defined as outpatient nirmatrelvir/ritonavir treatment within 5 days of symptom onset or coronavirus disease 2019 (COVID-19) diagnosis. Primary outcomes were maternal morbidity and mortality index (MMMI), all-cause maternal death and COVID-19-related hospitalization, while secondary outcomes were individual components of MMMI, preterm birth, stillbirth, neonatal death and cesarean section. One-to-ten propensity-score matching was conducted between nirmatrelvir/ritonavir users and nonusers, followed by cloning, censoring and weighting. Overall, 211 pregnant women on nirmatrelvir/ritonavir and 1,998 nonusers were included. Nirmatrelvir/ritonavir treatment was associated with reduced 28-day MMMI risk (absolute risk reduction (ARR) = 1.47%, 95% confidence interval (CI) = 0.21–2.34%) but not 28-days COVID-19-related hospitalization (ARR = −0.09%, 95% CI = −1.08% to 0.71%). Nirmatrelvir/ritonavir treatment was also associated with reduced risks of cesarean section (ARR = 1.58%, 95% CI = 0.85–2.39%) and preterm birth (ARR = 2.70%, 95% CI = 0.98–5.31%). No events of maternal or neonatal death or stillbirth were recorded. The findings suggest that nirmatrelvir/ritonavir is an effective treatment in symptomatic pregnant women with SARS-CoV-2 Omicron variant infection.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The clinical outcome data and vaccination records were extracted from the Hospital Authority database in Hong Kong, and data on confirmed cases of SARS-CoV-2 infection were extracted from the eSARS data provided by the Center for Health Protection (Department of Health, the Government of the Hong Kong Special Administrative Region). The data custodians (the Hospital Authority and the Department of Health) provided the underlying individual patient data to the University of Hong Kong to perform scientific research for the study. Restrictions apply to the availability of these data, which were used under the license of the Hospital Authority and the Department of Health for this study. The authors cannot transmit or release the data, in whole or in part in whatever form or media, or to any other parties or place outside Hong Kong. The authors fully comply with the duties under the laws of Hong Kong relating to the protection of personal data including those under the Personal Data (Privacy) Ordinance and its principles in all aspects.
Code availability
The code used for this study is publicly available on GitHub (https://github.com/MSH-Chung/COVID-19-pregnant-oral_antiviral).
References
Allotey, J. et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ 370, m3320 (2020).
Villar, J. et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr. 175, 817–826 (2021).
Oakes, M. C. et al. Pregnancy as a risk factor for severe coronavirus disease 2019 using standardized clinical criteria. Am. J. Obstet. Gynecol. MFM 3, 100319 (2021).
Jering, K. S. et al. Clinical characteristics and outcomes of hospitalized women giving birth with and without COVID-19. JAMA Intern. Med. 181, 714–717 (2021).
Adhikari, E. H. et al. COVID-19 cases and disease severity in pregnancy and neonatal positivity associated with Delta (B.1.617.2) and Omicron (B.1.1.529) variant predominance. JAMA 327, 1500–1502 (2022).
National Institutes of Health. Therapeutic management of nonhospitalized adults with COVID-19. https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults--therapeutic-management/ (2023).
World Health Organization. Therapeutics and COVID-19: living guideline, 13 January 2023. www.who.int/publications/i/item/WHO-2019-nCoV-therapeutics-2023.1 (2023).
Loza, A. et al. Short-term pregnancy outcomes after nirmatrelvir–ritonavir treatment for mild-to-moderate coronavirus disease 2019 (COVID-19). Obstet. Gynecol. 140, 447–449 (2022).
Garneau, W. M. et al. Analysis of clinical outcomes of pregnant patients treated with nirmatrelvir and ritonavir for acute SARS-CoV-2 infection. JAMA Netw. Open 5, e2244141 (2022).
Lin, C. Y. et al. Nirmatrelvir–ritonavir (Paxlovid) for mild coronavirus disease 2019 (COVID-19) in pregnancy and lactation. Obstet. Gynecol. 141, 957–960 (2023).
Villar, J. et al. Pregnancy outcomes and vaccine effectiveness during the period of omicron as the variant of concern, INTERCOVID-2022: a multinational, observational study. Lancet 401, 447–457 (2023).
Hammond, J. et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with COVID-19. N. Engl. J. Med. 386, 1397–1408 (2022).
Gbinigie, O. et al. Platform adaptive trial of novel antivirals for early treatment of COVID-19 in the community (PANORAMIC): protocol for a randomised, controlled, open-label, adaptive platform trial of community novel antiviral treatment of COVID-19 in people at increased risk of more severe disease. BMJ Open 13, e069176 (2023).
Conde-Agudelo, A. & Romero, R. SARS-CoV-2 infection during pregnancy and risk of preeclampsia: a systematic review and meta-analysis. Am. J. Obstet. Gynecol. 226, 68–89 (2022).
Lai, J. et al. SARS-CoV-2 and the subsequent development of preeclampsia and preterm birth: evidence of a dose-response relationship supporting causality. Am. J. Obstet. Gynecol. 225, 689–693 (2021).
Wei, S. Q., Bilodeau-Bertrand, M., Liu, S. & Auger, N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ 193, E540–E548 (2021).
National Institutes of Health. Pregnancy, lactation, and COVID-19 therapeutics. www.covid19treatmentguidelines.nih.gov/special-populations/pregnancy/pregnancy-lactation-and-covid-19-therapeutics/ (2023).
US Food and Drug Administration. Full prescribing information for Paxlovid. www.accessdata.fda.gov/drugsatfda_docs/label/2023/217188s000lbl.pdf (2023).
Chourasia, P. et al. Paxlovid (nirmatrelvir and ritonavir) use in pregnant and lactating woman: current evidence and practice guidelines—a scoping review. Vaccines 11, 107 (2023).
Hernán, M. A. & Robins, J. M. Using big data to emulate a target trial when a randomized trial is not available. Am. J. Epidemiol. 183, 758–764 (2016).
Hernán, M. A., Sauer, B. C., Hernández-Díaz, S., Platt, R. & Shrier, I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J. Clin. Epidemiol. 79, 70–75 (2016).
Wong, C. K. H. et al. Real-world effectiveness of early molnupiravir or nirmatrelvir–ritonavir in hospitalised patients with COVID-19 without supplemental oxygen requirement on admission during Hong Kong’s Omicron BA.2 wave: a retrospective cohort study. Lancet Infect. Dis. 22, 1681–1693 (2022).
Wong, C. K. H. et al. Real-world effectiveness of molnupiravir and nirmatrelvir plus ritonavir against mortality, hospitalisation, and in-hospital outcomes among community-dwelling, ambulatory patients with confirmed SARS-CoV-2 infection during the omicron wave in Hong Kong: an observational study. Lancet 400, 1213–1222 (2022).
Wong, C. K. H. et al. Viral burden rebound in hospitalised patients with COVID-19 receiving oral antivirals in Hong Kong: a population-wide retrospective cohort study. Lancet Infect. Dis. 23, 683–695 (2023).
HA Central Committee on Infectious Diseases and Emergency Response (CCIDER). Interim recommendation on clinical management of adult cases with coronavirus disease 2019 (COVID-19). Version 1.9 (2022).
Liverpool Drug Interactions Group. Interactions with outpatient medicines & nirmatrelvir/ritonavir (NMV/r). www.covid19-druginteractions.org/prescribing_resources/paxlovid-outpatient-medicines (2023).
Austin, P. C. Some methods of propensity-score matching had superior performance to others: results of an empirical investigation and Monte Carlo simulations. Biom. J. 51, 171–184 (2009).
Xie, Y., Bowe, B. & Al-Aly, Z. Molnupiravir and risk of hospital admission or death in adults with COVID-19: emulation of a randomized target trial using electronic health records. BMJ 380, e072705 (2023).
Maringe, C. et al. Reflection on modern methods: trial emulation in the presence of immortal-time bias. Assessing the benefit of major surgery for elderly lung cancer patients using observational data. Int. J. Epidemiol. 49, 1719–1729 (2020).
Willems, S. J. W., Schat, A., van Noorden, M. S. & Fiocco, M. Correcting for dependent censoring in routine outcome monitoring data by applying the inverse probability censoring weighted estimator. Stat. Methods Med. Res. 27, 323–335 (2016).
McMenamin, M. E. et al. Vaccine effectiveness of one, two, and three doses of BNT162b2 and CoronaVac against COVID-19 in Hong Kong: a population-based observational study. Lancet Infect. Dis. 22, 1435–1443 (2022).
Acknowledgements
This work was supported by the Health and Medical Research Fund (reference: COVID190210 to C.K.H.W.); Health Bureau, Government of Hong Kong Special Administrative Region, China; AIR@InnoHK administered by Innovation and Technology Commission, Government of Hong Kong Special Administrative Region, China. The funding source had no involvement in the study design, in the collection, analysis and interpretation of data, in the writing of the report or in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
C.K.H.W. designed the study, wrote the manuscript, contributed to the interpretation of the analysis and attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. C.K.H.W. and K.T.K.L. reviewed the literature and wrote the manuscript. Y.D. reviewed the literature and manuscript. M.S.H.C. and I.C.H.A conducted analysis and revised the manuscript. K.W.C. contributed to the interpretation of the analysis. C.K.H.W., M.S.H.C., I.C.H.A., and E.H.Y.L. accessed and verified the underlying data. E.H.Y.L, B.J.C. and G.M.L. reviewed and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
C.K.H.W. reports the receipt of the General Research Fund, Research Grant Council, Government of Hong Kong SAR; EuroQol Research Foundation; AstraZeneca and Boehringer Ingelheim, all outside the submitted work. B.J.C. reports honoraria from AstraZeneca, Fosun Pharma, GlaxoSmithKline, Haleon, Moderna, Pfizer, Roche and Sanofi Pasteur and has provided scientific advice to Pfizer and AstraZeneca on issues related to disease burden and vaccine effectiveness. He has not provided scientific advice to either company related to COVID-19 antiviral effectiveness, and he has not received any funding from Pfizer or AstraZeneca for any research on antiviral effectiveness including the current work. All other authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Kelly Gebo, Laurie Tomlinson, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Alison Farrell, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3, Supplementary Tables 1–6 and Supplementary Protocol.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wong, C.K.H., Lau, K.T.K., Chung, M.S.H. et al. Nirmatrelvir/ritonavir use in pregnant women with SARS-CoV-2 Omicron infection: a target trial emulation. Nat Med 30, 112–116 (2024). https://doi.org/10.1038/s41591-023-02674-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-023-02674-0
This article is cited by
-
Optimal timing of nirmatrelvir/ritonavir treatment after COVID-19 symptom onset or diagnosis: target trial emulation
Nature Communications (2023)