Abstract
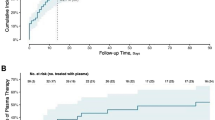
Despite advances, few therapeutics have shown efficacy in severe coronavirus disease 2019 (COVID-19). In a different context, virus-specific T cells have proven safe and effective. We conducted a randomized (2:1), open-label, phase 1/2 trial to evaluate the safety and efficacy of off-the-shelf, partially human leukocyte antigen (HLA)-matched, convalescent donor-derived severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-specific T cells (CoV-2-STs) in combination with standard of care (SoC) in patients with severe COVID-19 compared to SoC during Delta variant predominance. After a dose-escalated phase 1 safety study, 90 participants were randomized to receive CoV-2-ST+SoC (n = 60) or SoC only (n = 30). The co-primary objectives of the study were the composite of time to recovery and 30-d recovery rate and the in vivo expansion of CoV-2-STs in patients receiving CoV-2-ST+SoC over SoC. The key secondary objective was survival on day 60. CoV-2-ST+SoC treatment was safe and well tolerated. The study met the primary composite endpoint (CoV-2-ST+SoC versus SoC: recovery rate 65% versus 38%, P = 0.017; median recovery time 11 d versus not reached, P = 0.052, respectively; rate ratio for recovery 1.71 (95% confidence interval 1.03–2.83, P = 0.036)) and the co-primary objective of significant CoV-2-ST expansion compared to SοC (CoV-2-ST+SoC versus SoC, P = 0.047). Overall, in hospitalized patients with severe COVID-19, adoptive immunotherapy with CoV-2-STs was feasible and safe. Larger trials are needed to strengthen the preliminary evidence of clinical benefit in severe COVID-19. EudraCT identifier: 2021-001022-22.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
George Papanikolaou Hospital is committed to responsible and transparent sharing of clinical trial data with healthcare practitioners and researchers, toward the improvement of scientific knowledge and the promotion of innovative medical approaches. Participant de-identified data collected for this study, including text, tables, figures, appendices and documents, including the study protocol, statistical analysis plan and informed consent form, will be available after article publication. Researchers interested in obtaining access to documents and/or data for academic use only can make their request by submitting the scientific design, specific data needs and analysis and dissemination plans, which will be reviewed by the institutional review board of George Papanikolaou Hospital, and, based on scientific merit, data access could be granted. An agreement will be signed between the two parties stating that the data will be used only for the agreed purpose, in compliance with ethical and regulatory requirements and the commitments made to the study participants. Any publication derived from the accessed data should be of high quality, and George Papanikolaou Hospital’s institutional review board will have the right to review and comment on any draft manuscripts before publication.
Change history
27 July 2023
In the version of this article initially published, the Abstract did not list the EudraCT identifier: 2021-001022-22 (ClinicalTrials.gov ID: NCT05447013), which is now amended in the HTML and PDF versions of the article.
References
Hammond, J. et al. Oral nirmatrelvir for high-risk, nonhospitalized adults with Covid-19. N. Engl. J. Med. 386, 1397–1408 (2022).
Owen, D. R. et al. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science 374, 1586–1593 (2021).
Jayk Bernal, A. et al. Molnupiravir for oral treatment of Covid-19 in nonhospitalized patients. N. Engl. J. Med. 386, 509–520 (2022).
Gordon, A. et al. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N. Engl. J. Med. 384, 1491–1502 (2021).
Horby, P. et al. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 384, 693–704 (2021).
Beigel, J. H. et al. Remdesivir for the treatment of Covid-19—final report. N. Engl. J. Med. 383, 1813–1826 (2020).
Papadopoulou, A. et al. Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Sci. Transl. Med. 6, 242ra83 (2014).
Tzannou, I. et al. Off-the-shelf virus-specific T cells to treat BK virus, human herpesvirus 6, cytomegalovirus, Epstein–Barr virus, and adenovirus infections after allogeneic hematopoietic stem-cell transplantation. J. Clin. Oncol. 35, 3547–3557 (2017).
O’Reilly, R. J., Prockop, S., Hasan, A. N., Koehne, G. & Doubrovina, E. Virus-specific T-cell banks for ‘off the shelf’ adoptive therapy of refractory infections. Bone Marrow Transplant. 51, 1163–1172 (2016).
Jiang, W. et al. Pathogen-specific T cells beyond CMV, EBV and adenovirus. Curr. Hematol. Malig. Rep. 14, 247–260 (2019).
Kaeuferle, T., Krauss, R., Blaeschke, F., Willier, S. & Feuchtinger, T. Strategies of adoptive T-cell transfer to treat refractory viral infections post allogeneic stem cell transplantation. J. Hematol. Oncol. 12, 13 (2019).
Baugh, K. A., Tzannou, I. & Leen, A. M. Infusion of cytotoxic T lymphocytes for the treatment of viral infections in hematopoetic stem cell transplant patients. Curr. Opin. Infect. Dis. 31, 292–300 (2018).
Papadopoulou, A., Alvanou, M., Karavalakis, G., Tzannou, I. & Yannaki, E. Pathogen-specific T cells: targeting old enemies and new invaders in transplantation and beyond. Hemasphere 7, e809 (2023).
Kim, N. et al. Off-the-shelf partial HLA matching SARS-CoV-2 antigen specific T cell therapy: a new possibility for COVID-19 treatment. Front. Immunol. 12, 5562 (2021).
Keller, M. D. et al. SARS-CoV-2 specific T-cells are rapidly expanded for therapeutic use and target conserved regions of membrane protein. Blood 136, 2905–2917 (2020).
Kedzierska, K. & Thomas, P. G. Count on us: T cells in SARS-CoV-2 infection and vaccination. Cell Rep. Med. 3, 100562 (2022).
Ferreras, C. et al. SARS-CoV-2-specific memory T lymphocytes from COVID-19 convalescent donors: identification, biobanking, and large-scale production for adoptive cell therapy. Front. Cell Dev. Biol. 9, 293 (2021).
Cooper, R. S. et al. Rapid GMP-compliant expansion of SARS-CoV-2-specific T cells from convalescent donors for use as an allogeneic cell therapy for COVID-19. Front. Immunol. 11, 598402 (2021).
Leung, W. et al. Rapid production of clinical‐grade SARS‐CoV‐2 specific T cells. Adv. Cell Gene Ther. 3, e101 (2020).
Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 23, 186–193 (2022).
Papayanni, P.-G. et al. Vaccinated and convalescent donor–derived severe acute respiratory syndrome coronavirus 2-specific T cells as adoptive immunotherapy for high-risk coronavirus disease 2019 patients. Clin. Infect. Dis. 73, 2073–2082 (2021).
Anderson, B. E. et al. Memory CD4+ T cells do not induce graft-versus-host disease. J. Clin. Invest. 112, 101–108 (2003).
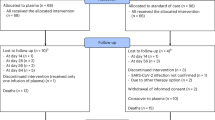
Schulz, K. F., Altman, D. G. & Moher, D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 340, 698–702 (2010).
Leisman, D. E. et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 8, 1233–1244 (2020).
Narni-Mancinelli, E. & Vivier, E. Clues that natural killer cells help to control COVID. Nature 600, 226–227 (2021).
Wei, R. et al. A landscape study on COVID-19 immunity at the single-cell level. Front. Immunol. 13, 918383 (2022).
Barouch, D. H. Covid-19 vaccines—immunity, variants, boosters. N. Engl. J. Med. 387, 1011–1020 (2022).
Antunez Muiños, P. J. et al. The COVID-19 lab score: an accurate dynamic tool to predict in-hospital outcomes in COVID-19 patients. Sci. Rep. 11, 9361 (2021).
Huang, I., Pranata, R., Lim, M. A., Oehadian, A. & Alisjahbana, B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Ther. Adv. Respir. Dis. 14, 1753466620937175 (2020).
Stone, J. H. et al. Efficacy of tocilizumab in patients hospitalized with Covid-19. N. Engl. J. Med. 383, 2333–2344 (2020).
Hermine, O. et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern. Med. 181, 32–40 (2021).
Cao, B. et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 382, 1787–1799 (2020).
Chen, G. et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J. Clin. Invest. 130, 2620–2629 (2020).
Zhou, R. et al. Acute SARS-CoV-2 infection impairs dendritic cell and T cell responses. Immunity 53, 864–877 (2020).
Yang, X. et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 8, 475–481 (2020).
Le Bert, N. et al. Highly functional virus-specific cellular immune response in asymptomatic SARS-CoV-2 infection. J. Exp. Med. 218, e20202617 (2021).
Rydyznski Moderbacher, C. et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell 183, 996 (2020).
Sekine, T. et al. Robust T cell immunity in convalescent individuals with asymptomatic or mild COVID-19. Cell 183, 158–168 (2020).
Tzannou, I. et al. Immunologic profiling of human metapneumovirus for the development of targeted immunotherapy. J. Infect. Dis. 216, 678–687 (2017).
McLaughlin, L. P. et al. Human parainfluenza virus-3 can be targeted by rapidly ex vivo expanded T-lymphocytes. Cytotherapy 18, 1515 (2016).
McKinstry, K. K. et al. Memory CD4+ T cells protect against influenza through multiple synergizing mechanisms. J. Clin. Invest. 122, 2847–2856 (2012).
Wilkinson, T. M. et al. Preexisting influenza-specific CD4+ T cells correlate with disease protection against influenza challenge in humans. Nat. Med. 18, 274–280 (2012).
Swain, S. L., McKinstry, K. K. & Strutt, T. M. Expanding roles for CD4+ T cells in immunity to viruses. Nat. Rev. Immunol. 12, 136–148 (2012).
Bollard, C. M. & Heslop, H. E. T cells for viral infections after allogeneic hematopoietic stem cell transplant. Blood 127, 3331–3340 (2016).
Vasileiou, S. et al. Allogeneic, off-the-shelf, SARS-CoV-2-specific T cells (ALVR109) for the treatment of COVID-19 in high-risk patients. Haematologica 108, 1840–1850 (2023).
Cruz, C. R. et al. Adverse events following infusion of T cells for adoptive immunotherapy: a 10-year experience. Cytotherapy 12, 743–749 (2010).
Papadopoulou, A. et al. Systemic inflammatory response syndrome after administration of unmodified T lymphocytes. Mol. Ther. 22, 1134–1138 (2014).
Bates, T. A. et al. Vaccination before or after SARS-CoV-2 infection leads to robust humoral response and antibodies that effectively neutralize variants. Sci. Immunol. 7, eabn8014 (2022).
Rodda, L. B. et al. Imprinted SARS-CoV-2-specific memory lymphocytes define hybrid immunity. Cell 185, 1588–1601 (2022).
Leen, A. M. et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood 121, 5113–5123 (2013).
Service, R. F. Bad news for Paxlovid? Resistance may be coming. Science 377, 138–139 (2022).
Iketani, S. et al. Multiple pathways for SARS-CoV-2 resistance to nirmatrelvir. Nature 613, 558–564 (2023).
Rubin, R. From positive to negative to positive again—the mystery of why COVID-19 rebounds in some patients who take Paxlovid. JAMA 327, 2380–2382 (2022).
US Food and Drug Administration. Clinical Considerations for Therapeutic Cancer Vaccines: Guidance for Industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/clinical-considerations-therapeutic-cancer-vaccines (2011).
Papadopoulou, A. et al. Clinical-scale production of Aspergillus-specific T cells for the treatment of invasive aspergillosis in the immunocompromised host. Bone Marrow Transpl. 54, 1963–1972 (2019).
Stallard, N. Optimal sample sizes for phase II clinical trials and pilot studies. Stat. Med. 31, 1031–1042 (2012).
Acknowledgements
We express our sincere thanks to the donors and patients. We thank all healthcare professionals who took care of the patients on hard times. This work was supported by the Committee of ‘Greece 2021’ and George Papanikolaou Hospital. We also greatly appreciate the support of community fundraising. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Study design and development of concept: A.P. and E.Y. GMP production and quality assurance: A.P., P.-G.P, A.G., I.V. and E.Y. Patient management: G.K., E.P., Z.B., M.K., E.V., V.P., A.V., E. Serasli, I.S., A.A, S.T., M.S., Z.S., N.K., M.B., M.T., D.A., G.P., M.D. and E.Y. Data collection: E.P., Z.B., A.V., M.G., F.S., A.X., A.F., P.-G.P., A.G., I.V., M.T., M.D., P.M., T.Κ., G.C., A.B., A.P. and M.G.K. Data analysis: G.S., G.K., G. Gounelas, G. Georgolopoulos, A.P. and E.Y. Study coordination: E.Y. and A.P. Obtained funding: E.Y. and A.A. Wrote the manuscript: A.P. and E.Y. All authors reviewed and revised the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Cliona Rooney, Katherine Kedzierska and Michael Schell for their contribution to the peer review of this work. Primary handling editor: Alison Farrell, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1
Kaplan-Meier curves for mortality (time to death) at 30 days in the modified intention-to-treat (mITT) population. P value was calculated using the two-sided log-rank test.
Extended Data Fig. 2
Subgroup analysis on mortality at 60 days, in the and mITT population. mITT: modified intention-to-treat analysis. Two-sided Wald test-based P values (as calculated using Cox regression). All P values shown are unadjusted for multiple testing and should therefore not be used to infer treatment effects.
Extended Data Fig. 3
Longitudinal analysis of T lymphocyte recovery kinetics over the study period in CoV-2-ST+SoC-treated (red lines, n = 58) and SoC-treated (blue lines, n = 29) patients. The thin lines represent individual CD3+ cell values for each patient. Bold lines are the quadratic fitted splines for each group. Shaded bands extend to 95% CI of the fitted values. Tukey HSD p-value is reported.
Extended Data Fig. 4
Correlation of the kinetics of circulating CoV-2-STs and viral load, in CoV-2-STs+SoC-treated (n = 57) and SoC-treated (n = 30) patients. The dotted lines represent the fitted values of circulating CoV-2-STs, the solid lines represent the fitted values of SARS-CoV-2 viral load. Shaded bands extend to 95% CI of fitted values. Pearson’s correlation coefficient values are reported.
Extended Data Fig. 5
Longitudinal trajectories of circulating CD4 (A) and CD8+ (B) cells in surviving (green lines, n = 58) and non-surviving (purple lines, n = 29) COVID-19 patients. Thin lines represent individual cell population counts for each patient. Bold lines are the quadratic fitted splines of each group. Shaded bands represent the 95% CI of the fitted values. One-way ANOVA p-values are reported for the survival status effect on both CD4 and CD8 trajectories.
Extended Data Fig. 6
Kaplan-Meier curve for hospitalization length at 60 days in the mITT population. P value was calculated using the two-sided log-rank test.
Supplementary information
Supplementary Information
Supplementary Figs. 1–15, Supplementary Tables 1–10, Supplementary References, Statistical Analysis Plan and Clinical Protocol.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Papadopoulou, A., Karavalakis, G., Papadopoulou, E. et al. SARS-CoV-2-specific T cell therapy for severe COVID-19: a randomized phase 1/2 trial. Nat Med 29, 2019–2029 (2023). https://doi.org/10.1038/s41591-023-02480-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-023-02480-8
This article is cited by
-
Immunomodulatory drugs have divergent effects on humoral and cellular immune responses to SARS-CoV-2 vaccination in people living with rheumatoid arthritis
Scientific Reports (2023)
-
Engineering immunosuppressive drug-resistant armored (IDRA) SARS-CoV-2 T cells for cell therapy
Cellular & Molecular Immunology (2023)