Abstract
In preclinical models, anakinra, an IL-1 receptor antagonist (IL-1Ra), reduced immune effector cell-associated neurotoxicity syndrome (ICANS) without compromising anti-CD19 chimeric antigen receptor (CAR) T-cell efficacy. We initiated a phase 2 clinical trial of anakinra in patients with relapsed/refractory large B-cell lymphoma and mantle cell lymphoma treated with commercial anti-CD19 CAR T-cell therapy. Here we report a non-prespecified interim analysis reporting the final results from cohort 1 in which patients received subcutaneous anakinra from day 2 until at least day 10 post-CAR T-cell infusion. The primary endpoint was the rate of severe (grade ≥3) ICANS. Key secondary endpoints included the rates of all-grade cytokine release syndrome (CRS) and ICANS and overall disease response. Among 31 treated patients, 74% received axicabtagene ciloleucel, 13% received brexucabtagene ciloleucel and 4% received tisagenlecleucel. All-grade ICANS occurred in 19%, and severe ICANS occurred in 9.7% of patients. There were no grade 4 or 5 ICANS events. All-grade CRS occurred in 74%, and severe CRS occurred in 6.4% of patients. The overall disease response rate was 77% with 65% complete response rate. These initial results show that prophylactic anakinra resulted in a low incidence of ICANS in patients with lymphoma receiving anti-CD19 CAR T-cell therapy and support further study of anakinra in immune-related neurotoxicity syndromes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
This trial is currently ongoing. Subject to patient privacy and confidentiality obligations, access to patient-level data and supporting clinical documents will be available upon request and subject to review by the study sponsor and/or the corresponding author on completion of the trial. Such requests can be made to the corresponding author by email at parkj6@mskcc.org. Any data and materials that can be shared will be released via a material transfer agreement and/or data access agreement.
References
Abramson, J. S. et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet 396, 839–852 (2020).
Neelapu, S. S. et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N. Engl. J. Med. 377, 2531–2544 (2017).
Schuster, S. J. et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 22, 1403–1415 (2021).
Wang, M. et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 382, 1331–1342 (2020).
Park, J. H. et al. Long-term follow-up of CD19 CAR therapy in acute lymphoblastic leukemia. N. Engl. J. Med. 378, 449–459 (2018).
Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018).
Porter, D. L., Levine, B. L., Kalos, M., Bagg, A. & June, C. H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 365, 725–733 (2011).
Lee, D. W. et al. ASTCT consensus grading for cytokine release syndrome and neurologic toxicity associated with immune effector cells. Biol. Blood Marrow Transplant. 25, 625–638 (2019).
Le, R. Q. et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist 23, 943–947 (2018).
Morris, E. C., Neelapu, S. S., Giavridis, T. & Sadelain, M. Cytokine release syndrome and associated neurotoxicity in cancer immunotherapy. Nat. Rev. Immunol. 22, 85–96 (2022).
Nastoupil, L. J. et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US Lymphoma CAR T consortium. J. Clin. Oncol. 38, 3119–3128 (2020).
Pennisi, M. et al. Comparing CAR T-cell toxicity grading systems: application of the ASTCT grading system and implications for management. Blood Adv. 4, 676–686 (2020).
Riedell, P. A. et al. Patterns of use, outcomes, and resource utilization among recipients of commercial axicabtagene ciloleucel and tisagenlecleucel for relapsed/refractory aggressive B cell lymphomas. Transpl. Cell Ther. 28, 669–676 (2022).
Locke, F. L. et al. Preliminary results of prophylactic tocilizumab after axicabtageneciloleucel (axi-cel; KTE-C19) treatment for patients with refractory, aggressive non-Hodgkin lymphoma (NHL). Blood 130, 1547 (2017).
Strati, P. et al. Prognostic impact of corticosteroids on efficacy of chimeric antigen receptor T-cell therapy in large B-cell lymphoma. Blood 137, 3272–3276 (2021).
Oluwole, O. O. et al. Prophylactic corticosteroid use in patients receiving axicabtagene ciloleucel for large B-cell lymphoma. Br. J. Haematol. 194, 690–700 (2021).
Topp, M. S. et al. Earlier corticosteroid use for adverse event management in patients receiving axicabtagene ciloleucel for large B-cell lymphoma. Br. J. Haematol. 195, 388–398 (2021).
Giavridis, T. et al. CAR T cell-induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat. Med. 24, 731–738 (2018).
Norelli, M. et al. Monocyte-derived IL-1 and IL-6 are differentially required for cytokine-release syndrome and neurotoxicity due to CAR T cells. Nat. Med. 24, 739–748 (2018).
Dinarello, C. A. Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27, 519–550 (2009).
Cheson, B. D. et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J. Clin. Oncol. 32, 3059–3068 (2014).
Pennisi, M. et al. Modified EASIX predicts severe cytokine release syndrome and neurotoxicity after chimeric antigen receptor T cells. Blood Adv. 5, 3397–3406 (2021).
Jung, S. H. & Kim, K. M. On the estimation of the binomial probability in multistage clinical trials. Stat. Med. 23, 881–896 (2004).
Koyama, T. & Chen, H. Proper inference from Simon’s two-stage designs. Stat. Med. 27, 3145–3154 (2008).
Santomasso, B. D. et al. Clinical and biological correlates of neurotoxicity associated with CAR T-cell therapy in patients with B-cell acute lymphoblastic leukemia. Cancer Discov. 8, 958–971 (2018).
Gust, J. et al. Endothelial activation and blood–brain barrier disruption in neurotoxicity after adoptive immunotherapy with CD19 CAR-T cells. Cancer Discov. 7, 1404–1419 (2017).
Gutierrez, E. G., Banks, W. A. & Kastin, A. J. Blood-borne interleukin-1 receptor antagonist crosses the blood-brain barrier. J. Neuroimmunol. 55, 153–160 (1994).
Strati, P. et al. Clinical efficacy of anakinra to mitigate CAR T-cell therapy-associated toxicity in large B-cell lymphoma. Blood Adv. 4, 3123–3127 (2020).
Wehrli, M. et al. Single-center experience using anakinra for steroid-refractory immune effector cell-associated neurotoxicity syndrome (ICANS). J. Immunother. Cancer 10, e003847 (2022).
Diorio, C. et al. Anakinra utilization in refractory pediatric CAR T-cell associated toxicities. Blood Adv. 6, 3398–3403 (2022).
Galea, J. et al. Intravenous anakinra can achieve experimentally effective concentrations in the central nervous system within a therapeutic time window: results of a dose-ranging study. J. Cereb. Blood Flow. Metab. 31, 439–447 (2011).
Gazeau, N. et al. Safety and efficacy of two anakinra dose regimens for refractory CRS or Icans after CAR T-cell therapy. Blood 138, 2816 (2021).
Oliai, C. et al. IL-1 receptor antagonist for prevention of severe immune effector cell-associated neurotoxicity syndrome. J. Clin. Oncol. 39, 7566 (2021).
Frigault, M. J. et al. A phase II trial of anakinra for the prevention of CAR-T cell mediated neurotoxicity. Blood 138, 2814 (2021).
Fong, Y., Huang, Y., Lemos, M. P. & McElrath, M. J. Rank-based two-sample tests for paired data with missing values. Biostatistics 19, 281–294 (2018).
Acknowledgements
This research was supported by the Geoffrey Beene Foundation Grant (to J.H.P.) and in part by NIH/NCI Cancer Center Support grant P30 CA008748. The drug supply (anakinra) was provided by Sobi. Sobi did not have any oversight of the trial and was not involved in writing the trial report. The content is solely the authors' responsibility and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Contributions
J.H.P. designed the initial study protocol. R.J.B., I.R. and M.S. provided support for the overall trial design. J.H.P., K.N., C.S., M.L.P., G.S., P.D., R.J.L., M.S., M.-A.P., R.S., A.A.T., E.M. and B.S. provided clinical care for the patients enrolled in the study. E.C. provided support for the clinical operation of the study. E.C., R.S. and A.A.T. provided support for data entry. A.H. performed tumor volume assessment. J.H.P., K.N. and S.D. performed the clinical data analysis and S.D. did the statistical analysis. J.H.P. and K.N. drafted the manuscript, and all authors have reviewed and participated in the revisions of the manuscript.
Corresponding author
Ethics declarations
Competing interests
J.H.P. received consulting fees from Affyimmune Therapeutics, Amgen, Autolus, Be Biopharma, Beigene, Bright Pharmaceutical Services, Inc., Curocel, Kite, Medpace, Minerva Biotechnologies, Pfizer, Servier, Sobi and Takeda; received honoraria from OncLive, Physician Education Resource, and MJH Life Sciences; serves on scientific advisory board of Allogene Therapeutics and Artiva Biotherapeutics; and received institutional research funding from Autolus, Genentech, Fate Therapeutics, InCyte, Servier and Takeda. C.S.S. has served as a paid consultant to Juno Therapeutics, Sanofi-Genzyme, Spectrum Pharmaceuticals, Novartis, Genmab, Precision Biosciences, Kite/a Gilead Company, Celgene/BMS, Gamida Cell, Karyopharm Therapeutics, MorphoSys, CSL Behring, Syncopation Life Sciences, CRISPR Therapeutics and GSK; serves on DSMBs for Ono Pharmaceuticals and CRISPR Therapeutics; and has received research funds for clinical trials from Juno Therapeutics, Celgene/BMS, Bristol Myers Squibb, Precision Biosciences, Actinium Pharmaceuticals, Sanofi-Genzyme and NKARTA. M.L.P. received consulting fees from BMS, Cellectar, Kite, Mustang Bio and Synthekine. G.S. received consulting fees from Amgen, BMS, Beyond Spring, and Janssen and serves on DSMB for Arcellx. P.D. received consulting fees from Kite. R.J.L. received consulting fees from Kite and Priothera. M.S. served as a paid consultant for McKinsey & Company, Angiocrine Bioscience, Inc., Kite and Omeros Corporation; received research funding from Angiocrine Bioscience, Inc., Omeros Corporation and Amgen, Inc.; and has received honoraria from i3Health and Medscape for CME-related activity. M.-A.P. reports personal fees from Adicet, Allovir, Caribou Biosciences, Celgene, BMS, Equilium, Exevir, Karyopharm, Merck, MorphoSys, Omeros, Syncopation, VectivBio AG, Vor Biopharma, Cidara Therapeutics, Medigene, Sellas Life Sciences and NexImmune; personal fees and other support from Incyte, Kite/Gilead, Miltenyi Biotec, Nektar Therapeutics and Novartis; and other support from OrcaBio outside the submitted work. B.S. is an inventor on United States Provisional Patent Application No. US20210181179A1 ‘Diagnosis and treatment of immunotherapy-induced neurotoxicity’ filed by Memorial Sloan Kettering Cancer Center and served in a consulting or advisory role for Celgene, Janssen, Legend Biotech, Incyte, Kite/Gilead and In8bio. A.H. is an owner/president of fMRI Consultants, LLC. R.J.B. has licensed intellectual property to and collected royalties from BMS, Caribou and Sanofi; received research funding from BMS; is a consultant to BMS, Atara Biotherapeutics Inc., CoImmune and Triumvira; was a consultant for Gracell Biotechnologies Inc but ended employment in the past 30 months; and is a member of the scientific advisory board for CoImmune and Triumvira. I.R. reports grants from Takeda Pharmaceuticals and Atara, personal fees from Mnemo Therapeutics, Akron, the Centre for Commercialization of Cancer and Oribiotech, and other support from Bristol Myers Squibb outside the submitted work. M.S. reports grants from Atara Biotherapeutics outside the submitted work, as well as patent 8389282 issued and licensed to Juno Therapeutics, patent 11242375 issued and licensed to Atara Biotherapeutics, patent 10370452 issued, licensed and with royalties paid from Fate Therapeutics, patent 11377637 issued and licensed to Takeda Pharmaceuticals, patent 11377637 issued and licensed to Mnemo Therapeutics, and patent 11377637 issued and licensed to Minerva Biotechnologies. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Medicine thanks Frederick Locke, Jongphil Kim and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary handling editor: Saheli Sadanand, in collaboration with the Nature Medicine team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
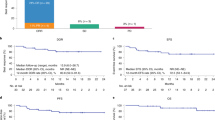
Extended Data Fig. 1 Response of patients with relapsed and refractory lymphoma.
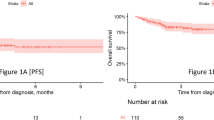
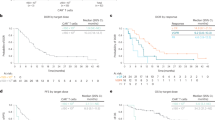
(2a) Best response by day 100 post CAR T-cell infusion displayed by the cell products received. (2b) Overall survival (2c) progression free survival of the study patients separated by LBCL vs. mantle cell lymphoma CAR denotes chimeric antigen receptor; ORR denotes overall response rate; PD denotes progressive disease; LBCL denotes large B-cell lymphoma; *One patient died of COVID-19 pneumonia at day 47 and did not have day 30 response assessment – this patient is included in the PD category.
Extended Data Fig. 2 Serum cytokine changes over time on study.
Changes in the level of serum cytokines after anakinra administration separated by grade 0–2 ICANS vs. grade 3–4 ICANS (n = 31).
Supplementary information
Supplementary Information
Supplementary Note—study protocol.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Park, J.H., Nath, K., Devlin, S.M. et al. CD19 CAR T-cell therapy and prophylactic anakinra in relapsed or refractory lymphoma: phase 2 trial interim results. Nat Med 29, 1710–1717 (2023). https://doi.org/10.1038/s41591-023-02404-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-023-02404-6