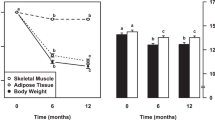
Abstract
The worldwide obesity epidemic1 makes it important to understand how lipid turnover (the capacity to store and remove lipids) regulates adipose tissue mass. Cross-sectional studies have shown that excess body fat is associated with decreased adipose lipid removal rates2,3. Whether lipid turnover is constant over the life span or changes during long-term weight increase or loss is unknown. We determined the turnover of fat cell lipids in adults followed for up to 16 years, by measuring the incorporation of nuclear bomb test-derived 14C in adipose tissue triglycerides. Lipid removal rate decreases during aging, with a failure to reciprocally adjust the rate of lipid uptake resulting in weight gain. Substantial weight loss is not driven by changes in lipid removal but by the rate of lipid uptake in adipose tissue. Furthermore, individuals with a low baseline lipid removal rate are more likely to remain weight-stable after weight loss. Therefore, lipid turnover adaptation might be important for maintaining pronounced weight loss. Together these findings identify adipose lipid turnover as an important factor for the long-term development of overweight/obesity and weight loss maintenance in humans.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
Afshin, A. et al. Health effects of overweight and obesity in 195 countries over 25 years. N. Engl. J. Med. 377, 13–27 (2017).
Arner, P. et al. Dynamics of human adipose lipid turnover in health and metabolic disease. Nature 478, 110–113 (2011).
Rydén, M., Andersson, D. P., Bernard, S., Spalding, K. & Arner, P. Adipocyte triglyceride turnover and lipolysis in lean and overweight subjects. J. Lipid Res. 54, 2909–2913 (2013).
Eto, H. et al. Characterization of structure and cellular components of aspirated and excised adipose tissue. Plast. Reconstr. Surg. 124, 1087–1097 (2009).
Klein, R. A., Halliday, D. & Pittet, P. G. The use of 13-methyltetradecanoic acid as an indicator of adipose tissue turnover. Lipids 15, 572–579 (1980).
Mårin, P., Rebuffé-Scrive, M. & Björntorp, P. Uptake of triglyceride fatty acids in adipose tissue in vivo in man. Eur. J. Clin. Invest. 20, 158–165 (1990).
Mårin, P., Odén, B. & Björntorp, P. Assimilation and mobilization of triglycerides in subcutaneous abdominal and femoral adipose tissue in vivo in men: effects of androgens. J. Clin. Endocrinol. Metab. 80, 239–243 (1995).
Strawford, A., Antelo, F., Christiansen, M. & Hellerstein, M. K. Adipose tissue triglyceride turnover, de novo lipogenesis, and cell proliferation in humans measured with 2H2O. Am. J. Physiol. Endocrinol. Metab. 286, E577–E588 (2004).
Guillermier, C. et al. Imaging mass spectrometry demonstrates age-related decline in human adipose plasticity. JCI Insight 2, e90349 (2017).
Arner, P., Andersson, D. P., Bäckdahl, J., Dahlman, I. & Rydén, M. Weight gain and impaired glucose metabolism in women are predicted by inefficient subcutaneous fat cell lipolysis. Cell Metab. 28, 45–54.e43 (2018).
Spalding, K. L. et al. Impact of fat mass and distribution on lipid turnover in human adipose tissue. Nat. Commun. 8, 15253 (2017).
Nydal, R. & Lövseth, K. Distribution of radiocarbon from nuclear tests. Nature 206, 1029–1031 (1965).
Levin, I. & Kromer, B. The tropospheric 14CO2 level in mid-latitudes of the Northern Hemisphere (1959–2003). Radiocarbon 46, 1261–1272 (2004).
Levin, I., Kromer, B. & Hammer, S. Atmospheric Δ14CO2 trend in Western European background air from 2000 to 2012.Tellus B Chem. Phys. Meteorol. 65, 20092 (2013).
Hammer, F. & Levin, I. Monthly mean atmospheric Δ12CO2 at Jungfraujoch and Schauinsland from 1986 to 2016. https://doi.org/10.11588/data/10100 (2017).
Frayn, K., Bernard, S., Spalding, K. & Arner, P. Adipocyte triglyceride turnover is independently associated with atherogenic dyslipidemia. J. Am. Heart Assoc. 1, e003467 (2012).
Després, J. P. et al. The effect of a 20-week endurance training program on adipose-tissue morphology and lipolysis in men and women. Metab. Clin. Exp. 33, 235–239 (1984).
Kather, H. et al. Influences of variation in total energy intake and dietary composition on regulation of fat cell lipolysis in ideal-weight subjects. J. Clin. Invest. 80, 566–572 (1987).
Swinburn, B., Sacks, G. & Ravussin, E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. Am. J. Clin. Nutr. 90, 1453–1456 (2009).
Arner, E., Rydén, M. & Arner, P. Tumor necrosis factor alpha and regulation of adipose tissue. N. Engl. J. Med. 362, 1151–1153 (2010).
Santosa, S. & Jensen, M. D. The sexual dimorphism of lipid kinetics in humans. Front. Endocrinol. (Lausanne) 6, 103 (2015).
De Glisezinski, I. et al. Endurance training changes in lipolytic responsiveness of obese adipose tissue. Am. J. Physiol. 275, E951–E956 (1998).
You, T., Berman, D. M., Ryan, A. S. & Nicklas, B. J. Effects of hypocaloric diet and exercise training on inflammation and adipocyte lipolysis in obese postmenopausal women. J. Clin. Endocrinol. Metab. 89, 1739–1746 (2004).
Seals, D. R. & Esler, M. D. Human ageing and the sympathoadrenal system. J. Physiol. 528, 407–417 (2000).
Lönnqvist, F., Nyberg, B., Wahrenberg, H. & Arner, P. Catecholamine-induced lipolysis in adipose tissue of the elderly. J. Clin. Invest. 85, 1614–1621 (1990).
Dulloo, A. G., Jacquet, J., Montani, J. P. & Schutz, Y. Adaptive thermogenesis in human body weight regulation: more of a concept than a measurable entity? Obes. Rev. 13, 105–121 (2012).
Ravussin, E. & Ryan, D. H. Energy expenditure and weight control: is the biggest loser the best loser? Obesity (Silver Spring) 24, 1607–1608 (2016).
Rosenbaum, M. & Leibel, R. L. Models of energy homeostasis in response to maintenance of reduced body weight. Obesity (Silver Spring) 24, 1620–1629 (2016).
Hall, K. D. et al. Quantification of the effect of energy imbalance on bodyweight. Lancet 378, 826–837 (2011).
Linné, Y., Dahlman, I. & Hoffstedt, J. β1-Adrenoceptor gene polymorphism predicts long-term changes in body weight. Int. J. Obes. 29, 458–462 (2005).
Hoffstedt, J. et al. Regional impact of adipose tissue morphology on the metabolic profile in morbid obesity. Diabetologia 53, 2496–2503 (2010).
Hoffstedt, J. et al. Long-term protective changes in adipose tissue after gastric bypass. Diabetes Care 40, 77–84 (2017).
Andersson, D. P., Arner, E., Hogling, D. E., Rydén, M. & Arner, P. Abdominal subcutaneous adipose tissue cellularity in men and women. Int. J. Obes. 41, 1564–1569 (2017).
Bedogni, G. et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 6, 33 (2006).
Henderson, M. & Freeman, C. P. A self-rating scale for bulimia. The ‘BITE’. Br. J. Psychiatry 150, 18–24 (1987).
Salehpour, M., Håkansson, K. & Possnert, G. Accelerator mass spectrometry of ultra-small samples with applications in the biosciences. Nucl. Instrum. Methods Phys. Res. B 294, 97–103 (2013).
Salehpour, M., Håkansson, K., Possnert, G., Wacker, L. & Synal, H. A. Performance report for the low energy compact accelerator mass spectrometer at Uppsala University. Nucl. Instrum. Methods Phys. Res. B 371, 360–364 (2016).
Stuiver, M. & Polach, H. A. Discussion reporting of 14C data. Radiocarbon 19, 355–363 (1977).
Reimer, P. J., Brown, T. A. & Reimer, R. W. Discussion: reporting and calibration of post-bomb 14C data. Radiocarbon 46, 1299–1304 (2004).
Bernard, S., Frisén, J. & Spalding, K. L. A mathematical model for the interpretation of nuclear bomb test derived 14C incorporation in biological systems. Nucl. Instrum. Methods Phys. Res. B 268, 1295–1298 (2010).
Vors, C. et al. Modulating absorption and postprandial handling of dietary fatty acids by structuring fat in the meal: a randomized crossover clinical trial. Am. J. Clin. Nutr. 97, 23–36 (2013).
U.S. Department of Health and Human Services and U.S. Department of Agriculture. Dietary Guidelines for Americans 2015–2020 8th edn. https://health.gov/dietaryguidelines/2015/guidelines/ (2015).
Jeukendrup, A. E., Saris, W. H. & Wagenmakers, A. J. Fat metabolism during exercise: a review. Part I: fatty acid mobilization and muscle metabolism. Int. J. Sports Med. 19, 231–244 (1998).
Acknowledgements
We would like to acknowledge the assistance of research nurses, Y. Widlund and K. Hertel, as well as laboratory technician K. Wåhlén. This study was supported by grants from the Stockholm County Council (no. ALF SLL20160040 to M.R. and CIMED project code no. 3115 to P.A.), Swedish Research Council (no. K2014-54×−14510-12-5 to M.R., no. K2012-55×-01034-46-5 to P.A. and no. 542-2013-8358 to K.S.), the Strategic Research Program for Diabetes at Karolinska Institutet (no. H721309942 to M.R., no. H721105932 to P.A. and no. C5471152 to K.S.), the Novo Nordisk Foundation (no. 120C101637 to P.A., the Tripartite Immunometabolism Consortium no. NNF15CC0018486 and MSAM consortium no. NNF15SA0018346 to M.R. and an Excellence Project Award no. NNF12OC1016064 to K.S.), the Swedish Diabetes Foundation (no. DIA2016-097 to M.R.), Karolinska Institutet/Astra Zeneca Integrated Cardiometabolic Centre (no. H725701603 to K.S.) and the Vallee Foundation Vallee Scholar Award (no. C5471234 to K.S.). D.P.A. was supported by The Swedish Society of Medicine (no. H721748513) and the Stockholm County Council (no. K0138-2015). A.T. was supported by The Erling-Persson Family foundation (project code no. 140604). S.B. was supported by the Institut rhônalpin des systèmes complexes (project code no. 12554).
Author information
Authors and Affiliations
Contributions
K.L.S. and P.A. designed the study. A.T. recruited the patients. P.A., D.P.A. and M.R. examined the patients. L.A. and K.-Y.F. prepared the adipose samples. M.S. performed the 14C AMS measurements. S.B. performed the mathematical modeling. K.L.S., P.A., S.B. and M.R. analyzed the data. K.L.S., P.A., M.R. and S.B. wrote the first version of the paper. All authors contributed and approved the final version of the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information: Jennifer Sargent and Joao Monteiro were the primary editors on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Clinical data on the examined cohorts.
Values are the mean ± s.d. and (range). Conditions are compared using a paired t-test. Sex distribution between weight groups had a P value of 0.74 in cohort 1 by Fisher’s exact test. Cohort 2 was composed of women only. Body fat was measured with bioimpedance. See Methods for a definition of weight groups in cohort 1.
Extended Data Fig. 2 Relationship between changes in lipid and participant age over time.
Cohort 1 was investigated twice with approximately a 13-year interval. The open circles are the first (baseline) and the closed circles the second (follow-up) examination. A large interindividual variation was observed. Despite this, lipid age increased in 42 out of 54 participants examined (P < 0.0001 by two-sample paired sign test).
Extended Data Fig. 3 Simulation of lipid dynamics.
Simulation of lipid dynamics (see and equation (13)) with estimated Kin and Kout for 33 individuals in cohort 2 for whom all data were available. Simulations (gray) closely follow the estimated lipid age (red) if estimates are consistent with the equilibrium assumption. For nine individuals, the simulation deviated significantly, indicating that the equilibrium assumption might not hold, leading to underestimating the true removal rate (and overestimating the true Kin).
Extended Data Fig. 4 Relationship between lipid age at first examination and changes in body composition over time (cohort 2).
Relationship between lipid age at first examination and changes (second minus first examination) in body composition over time (cohort 2). a, Percentage change in BMI. b, Changes (Δ) in total fat mass determined by DEXA. c, Changes (Δ) in abdominal subcutaneous fat mass corresponding to the site of adipose biopsy (ESAT). Cohort 2 was examined by linear regression. The number of individuals (n) are indicated. See Methods for further details.
Extended Data Fig. 5 Relationship between changes in lipid age and FLI or EVAT mass in cohort 2.
a,b, Relationship between changes (Δ, second minus first examination) in lipid age and FLI (a) or EVAT (b) in cohort 2. Cohort 2 was examined using linear regression. The number of individuals (n) are indicated. See Methods for further details.
Extended Data Fig. 6 Relationship between changes in eating behavior and lipid age in cohort 2.
Relationship between changes (Δ, second minus first examination) in eating behavior and lipid age in cohort 2. a,b, The questionnaire on eating behavior (BITE) was used and is detailed in the Methods. BITE-A, magnitude of symptoms; BITE-B, severity of symptoms. Data were examined using linear regression; r and P values are shown. The number of individuals (n) are indicated.
Extended Data Fig. 7 Relationship between lipid age and measures of indirect calorimetry at first examination.
a, Data for resting energy expenditure. b, Data for respiratory quotient. Values for both cohorts combined were subjected to linear regression analysis. n = 51 and 41 for cohorts 1 and 2, respectively. r and P values are shown. When the cohorts were analyzed separately, the correlation parameters were r = 0.02–0.12 (a) and P = 0.45–0.89 (b). Data were examined by linear regression; r and P values are shown. The number of individuals (n) are indicated.
Supplementary information
Supplementary Information
Supplementary Tables 1–4
Rights and permissions
About this article
Cite this article
Arner, P., Bernard, S., Appelsved, L. et al. Adipose lipid turnover and long-term changes in body weight. Nat Med 25, 1385–1389 (2019). https://doi.org/10.1038/s41591-019-0565-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-019-0565-5
This article is cited by
-
Futile lipid cycling: from biochemistry to physiology
Nature Metabolism (2024)
-
A Lifshitz–Slyozov type model for adipocyte size dynamics: limit from Becker–Döring system and numerical simulation
Journal of Mathematical Biology (2024)
-
Lipid cycling isn’t all futile
Nature Metabolism (2023)
-
The long noncoding RNA ADIPINT regulates human adipocyte metabolism via pyruvate carboxylase
Nature Communications (2022)
-
Time-of-day dependent effect of proanthocyanidins on adipose tissue metabolism in rats with diet-induced obesity
International Journal of Obesity (2022)