Abstract
CD4+ T helper 17 (TH17) cells protect barrier tissues but also trigger autoimmunity. The mechanisms behind these opposing processes remain unclear. Here, we found that the transcription factor EGR2 controlled the transcriptional program of pathogenic TH17 cells in the central nervous system (CNS) but not that of protective TH17 cells at barrier sites. EGR2 was significantly elevated in myelin-reactive CD4+ T cells from patients with multiple sclerosis and mice with autoimmune neuroinflammation. The EGR2 transcriptional program was intricately woven within the TH17 cell transcriptional regulatory network and showed high interconnectivity with core TH17 cell-specific transcription factors. Mechanistically, EGR2 enhanced TH17 cell differentiation and myeloid cell recruitment to the CNS by upregulating pathogenesis-associated genes and myelomonocytic chemokines. T cell-specific deletion of Egr2 attenuated neuroinflammation without compromising the host’s ability to control infections. Our study shows that EGR2 regulates tissue-specific and disease-specific functions in pathogenic TH17 cells in the CNS.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request. There are no restrictions on data availability. Raw and processed data have been deposited at the GEO repository under the following accession numbers: GSE168288 (RNA-seq), GSE224960 (FastATAC-seq) and GSE226795 (CUT&Tag).
Code availability
No custom-made code was used in the analysis. The pipelines for analysis can be obtained by e-mailing alejandro.villarino@miami.edu (RNA-seq), hiroyuki.nagashima@nih.gov (FastATAC-seq) and daniel.chauss@nih.gov (CUT&Tag).
References
Bettelli, E. et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature 441, 235–238 (2006).
Mangan, P. R. et al. Transforming growth factor-β induces development of the TH17 lineage. Nature 441, 231–234 (2006).
Veldhoen, M., Hocking, R. J., Atkins, C. J., Locksley, R. M. & Stockinger, B. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity 24, 179–189 (2006).
Ghoreschi, K. et al. Generation of pathogenic TH17 cells in the absence of TGF-β signalling. Nature 467, 967–971 (2010).
Langrish, C. L. et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201, 233–240 (2005).
Lee, Y. et al. Induction and molecular signature of pathogenic TH17 cells. Nat. Immunol. 13, 991–999 (2012).
McGeachy, M. J. et al. TGF-β and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain TH-17 cell-mediated pathology. Nat. Immunol. 8, 1390–1397 (2007).
McGeachy, M. J. et al. The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 10, 314–324 (2009).
Kleinschek, M. A. et al. Circulating and gut-resident human Th17 cells express CD161 and promote intestinal inflammation. J. Exp. Med. 206, 525–534 (2009).
Nistala, K. et al. Th17 plasticity in human autoimmune arthritis is driven by the inflammatory environment. Proc. Natl Acad. Sci. USA 107, 14751–14756 (2010).
Tzartos, J. S. et al. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am. J. Pathol. 172, 146–155 (2008).
Zheng, Y. et al. Interleukin-22, a TH17 cytokine, mediates IL-23-induced dermal inflammation and acanthosis. Nature 445, 648–651 (2007).
Hueber, W. et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61, 1693–1700 (2012).
Lee, J. S. et al. Interleukin-23-independent IL-17 production regulates intestinal epithelial permeability. Immunity 43, 727–738 (2015).
O’Connor, W. Jr. et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat. Immunol. 10, 603–609 (2009).
Gaublomme, J. T. et al. Single-cell genomics unveils critical regulators of Th17 cell pathogenicity. Cell 163, 1400–1412 (2015).
Ciofani, M. et al. A validated regulatory network for Th17 cell specification. Cell 151, 289–303 (2012).
Yosef, N. et al. Dynamic regulatory network controlling TH17 cell differentiation. Nature 496, 461–468 (2013).
Myouzen, K. et al. Regulatory polymorphisms in EGR2 are associated with susceptibility to systemic lupus erythematosus. Hum. Mol. Genet 19, 2313–2320 (2010).
Riveros, C. et al. A transcription factor map as revealed by a genome-wide gene expression analysis of whole-blood mRNA transcriptome in multiple sclerosis. PLoS ONE 5, e14176 (2010).
Rioux, J. D. et al. Genome-wide association study identifies new susceptibility loci for Crohn disease and implicates autophagy in disease pathogenesis. Nat. Genet. 39, 596–604 (2007).
Cao, Y. et al. Functional inflammatory profiles distinguish myelin-reactive T cells from patients with multiple sclerosis. Sci. Transl. Med. 7, 287ra274 (2015).
Hirota, K. et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat. Immunol. 12, 255–263 (2011).
Miao, T. et al. Early growth response gene-2 controls IL-17 expression and Th17 differentiation by negatively regulating Batf. J. Immunol. 190, 58–65 (2013).
Lauritsen, J. P. et al. Egr2 is required for Bcl-2 induction during positive selection. J. Immunol. 181, 7778–7785 (2008).
Lawson, V. J., Weston, K. & Maurice, D. Early growth response 2 regulates the survival of thymocytes during positive selection. Eur. J. Immunol. 40, 232–241 (2010).
Du, N. et al. EGR2 is critical for peripheral naive T-cell differentiation and the T-cell response to influenza. Proc. Natl Acad. Sci. USA 111, 16484–16489 (2014).
Zhu, B. et al. Early growth response gene 2 (Egr-2) controls the self-tolerance of T cells and prevents the development of lupuslike autoimmune disease. J. Exp. Med. 205, 2295–2307 (2008).
Taillebourg, E., Buart, S. & Charnay, P. Conditional, floxed allele of the Krox20 gene. Genesis 32, 112–113 (2002).
Vacchio, M. S. et al. A ThPOK-LRF transcriptional node maintains the integrity and effector potential of post-thymic CD4+ T cells. Nat. Immunol. 15, 947–956 (2014).
Carleton, M. et al. Early growth response transcription factors are required for development of CD4-CD8- thymocytes to the CD4+CD8+ stage. J. Immunol. 168, 1649–1658 (2002).
Shao, H., Kono, D. H., Chen, L. Y., Rubin, E. M. & Kaye, J. Induction of the early growth response (Egr) family of transcription factors during thymic selection. J. Exp. Med. 185, 731–744 (1997).
Jager, A., Dardalhon, V., Sobel, R. A., Bettelli, E. & Kuchroo, V. K. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J. Immunol. 183, 7169–7177 (2009).
Chung, Y. et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity 30, 576–587 (2009).
Wang, Y. et al. The transcription factors T-bet and Runx are required for the ontogeny of pathogenic interferon-γ-producing T helper 17 cells. Immunity 40, 355–366 (2014).
Yang, Y. et al. T-bet is essential for encephalitogenicity of both Th1 and Th17 cells. J. Exp. Med. 206, 1549–1564 (2009).
Lee, Y. K. et al. Late developmental plasticity in the T helper 17 lineage. Immunity 30, 92–107 (2009).
Ivanov, I. I. et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell 139, 485–498 (2009).
Omenetti, S. et al. The intestine harbors functionally distinct homeostatic tissue-resident and inflammatory Th17 cells. Immunity 51, 77–89.e76 (2019).
Conti, H. R. et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. J. Exp. Med. 206, 299–311 (2009).
Lazarevic, V. et al. The gene encoding early growth response 2, a target of the transcription factor NFAT, is required for the development and maturation of natural killer T cells. Nat. Immunol. 10, 306–313 (2009).
Tanaka, A. et al. Construction of a T cell receptor signaling range for spontaneous development of autoimmune disease. J. Exp. Med. 220, e20220386 (2023).
Mufazalov, I. A. et al. IL-1 signaling is critical for expansion but not generation of autoreactive GM-CSF+ Th17 cells. EMBO J. 36, 102–115 (2017).
Suzuki, Y., Orellana, M. A., Schreiber, R. D. & Remington, J. S. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science 240, 516–518 (1988).
Yednock, T. A. et al. Prevention of experimental autoimmune encephalomyelitis by antibodies against α4βl integrin. Nature 356, 63–66 (1992).
Wang, Y. et al. A critical role of LFA-1 in the development of Th17 cells and induction of experimental autoimmune encephalomyelytis. Biochem. Biophys. Res. Commun. 353, 857–862 (2007).
Rothhammer, V. et al. Th17 lymphocytes traffic to the central nervous system independently of α4 integrin expression during EAE. J. Exp. Med. 208, 2465–2476 (2011).
Guerrini, M. M. et al. Inhibition of the TNF family cytokine RANKL prevents autoimmune inflammation in the central nervous system. Immunity 43, 1174–1185 (2015).
Reboldi, A. et al. C-C chemokine receptor 6-regulated entry of TH-17 cells into the CNS through the choroid plexus is required for the initiation of EAE. Nat. Immunol. 10, 514–523 (2009).
Bettelli, E. et al. Myelin oligodendrocyte glycoprotein-specific T cell receptor transgenic mice develop spontaneous autoimmune optic neuritis. J. Exp. Med. 197, 1073–1081 (2003).
Topilko, P. et al. Multiple pituitary and ovarian defects in Krox-24 (NGFI-A, Egr-1)-targeted mice. Mol. Endocrinol. 12, 107–122 (1998).
Tourtellotte, W. G. & Milbrandt, J. Sensory ataxia and muscle spindle agenesis in mice lacking the transcription factor Egr3. Nat. Genet. 20, 87–91 (1998).
Zhu, J. et al. The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. Immunity 37, 660–673 (2012).
Eberl, G. et al. An essential function for the nuclear receptor RORγt in the generation of fetal lymphoid tissue inducer cells. Nat. Immunol. 5, 64–73 (2004).
Chao, D. T. et al. Bcl-XL and Bcl-2 repress a common pathway of cell death. J. Exp. Med. 182, 821–828 (1995).
Rogers, P. R., Dubey, C. & Swain, S. L. Qualitative changes accompany memory T cell generation: faster, more effective responses at lower doses of antigen. J. Immunol. 164, 2338–2346 (2000).
Rogers, P. R., Grey, H. M. & Croft, M. Modulation of naive CD4 T cell activation with altered peptide ligands: the nature of the peptide and presentation in the context of costimulation are critical for a sustained response. J. Immunol. 160, 3698–3704 (1998).
Kwong, B. et al. T-bet-dependent NKp46+ innate lymphoid cells regulate the onset of TH17-induced neuroinflammation. Nat. Immunol. 18, 1117–1127 (2017).
Bouladoux, N., Harrison, O. J. & Belkaid, Y. The mouse model of infection with Citrobacter rodentium. Curr. Protoc. Immunol. 119, 19.15.11–19.15.25 (2017).
Oldenhove, G. et al. Decrease of Foxp3+ Treg cell number and acquisition of effector cell phenotype during lethal infection. Immunity 31, 772–786 (2009).
Solis, N. V. & Filler, S. G. Mouse model of oropharyngeal candidiasis. Nat. Protoc. 7, 637–642 (2012).
Break, T. J. et al. Aberrant type 1 immunity drives susceptibility to mucosal fungal infections. Science 371, eaay5731 (2021).
Rau, A., Gallopin, M., Celeux, G. & Jaffrezic, F. Data-based filtering for replicated high-throughput transcriptome sequencing experiments. Bioinformatics 29, 2146–2152 (2013).
Anders, S. et al. Count-based differential expression analysis of RNA sequencing data using R and Bioconductor. Nat. Protoc. 8, 1765–1786 (2013).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Corces, M. R. et al. Lineage-specific and single-cell chromatin accessibility charts human hematopoiesis and leukemia evolution. Nat. Genet. 48, 1193–1203 (2016).
Langmead, B., Trapnell, C., Pop, M. & Salzberg, S. L. Ultrafast and memory-efficient alignment of short DNA sequences to the human genome. Genome Biol. 10, R25 (2009).
Xu, H. et al. FastUniq: a fast de novo duplicates removal tool for paired short reads. PLoS ONE 7, e52249 (2012).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Heinz, S. et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol. Cell 38, 576–589 (2010).
Kolde, R. pheatmap: Pretty Heatmaps. R version 4.2.2.2 (2018).
Zhu, Q., Liu, N., Orkin, S. H. & Yuan, G. C. CUT&RUNTools: a flexible pipeline for CUT&RUN processing and footprint analysis. Genome Biol. 20, 192 (2019).
Meers, M. P., Tenenbaum, D. & Henikoff, S. Peak calling by Sparse Enrichment Analysis for CUT&RUN chromatin profiling. Epigenetics Chromatin 12, 42 (2019).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Acknowledgements
This research was supported by the Intramural Research Program of the National Institutes of Health (NIH) NCI, Center for Cancer Research (ZIA BC011765), National Institute of Allergy and Infectious Diseases (ZIA AI001175), National Institute of Diabetes and Digestive and Kidney Diseases (ZIA DK075149 to B.A.), National Heart, Lung, and Blood Institute, and National Institute of Arthritis and Musculoskeletal and Skin Diseases. We thank R. Germain, R. Bosselut, A. Singer and D. Hafler for scientific discussions and critical reading of the manuscript; all members of the NCI (EIB) flow cytometry core facility, S. Sharrow, A. Crossman, L. Granger and T. Adams for expert technical help with flow cytometry and cell sorting; and the members of the CCR Sequencing Facility at the Frederick National Laboratory for Cancer Research for help during sample preparation, sequencing and data processing. Special thanks to members of the NIAMS Sequencing Core Facility (S. Dell’Orso and F. Naz) and the NIAMS Bioinformatics laboratory (Biodata Mining and Discovery Section), H.-W. Sun, K. Jiang and A. Uhlman. This work used the computational resources of the NIH High‐Performance Computing Biowulf Cluster. We are grateful to M. Lu for technical assistance and genotyping.
Author information
Authors and Affiliations
Contributions
Y.Y.G. and Y.W. performed experiments, analyzed data and contributed equally to this work. A.V.V., V.M.L. and V.N.K. performed bioinformatics analysis of RNA-seq data. D.C. and B.A. performed CUT&Tag and analyzed the results. H.N. performed FastATAC-seq and analyzed the results. C.A.S. performed immunofluorescence staining of CNS tissue. N.B. helped with C. rodentium and T. gondii infections and analysis. T.J.B. and M.S.A. performed C. albicans experiments. L.B.C. contributed to the optimization of the ChIP protocol for TH17 cells. M.S.L., J.J.O., J.D.P., J.H.P., J.Z., D.L.W. and W.J.L. contributed new reagents and/or analytical tools. Y.B. helped with manuscript writing and editing. V.L. conceived the research, designed experiments, performed experiments, analyzed data and wrote the manuscript. All authors contributed to the editing of the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Brigitta Stockinger and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Primary Handling Editor: Ioana Visan, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
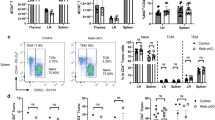
Extended Data Fig. 1 EGR2 reinforces TH17 differentiation program in a RORγt-dependent manner.
a, Representative flow plots showing the frequencies of RORγt- and IL-17A-expressing 2D2 TH17(β,6,23) cells before the adoptive transfer. Data are representative of n = 3 independent experiments. b, Quantitative RT-PCR analysis of Egr1, Egr2, Egr3, and Egr4 mRNA expression in pathogenic 2D2 WT CD4+ T cells from the CNS of Tcrb−/− mice that received 2D2 WT TH17(β,6,23) cells (20 days post-transfer). Box plot depicts median (line), lower and upper quartiles. Data represent biologically independent replicates from n = 3 independent experiments. c, Quantitative RT-PCR analysis of Egr1 and Egr2 mRNA expression in sorted YFP+ and YFP− CD4+ T cell populations isolated from the spleen and CNS of MOG35-55 immunized Il17a-Cre R26ReYFP fate-mapping mice. Data are presented as the log2 fold-change in the relative expression of Egr1 and Egr2 in YFP+ over YFP− CD4+ T cells. Data represent biologically independent replicates from n = 2 independent experiments. d, Quantitative RT-PCR analysis of Rorc, Il17a, Il17f, Il21 and Il22 mRNA in TH17 (β,6) cells transduced with empty virus (EV-RV), or retroviruses expressing Egr1 (Egr1-RV) or Egr2 (Egr2-RV). Mean values ± s.e.m. are reported. Data represent biologically independent replicates from n = 6 independent experiments. ****P < 0.0001, **P < 0.01, *P < 0.05; two-tailed Student’s t test.
Extended Data Fig. 2 EGRs function redundantly during TH17 cell differentiation.
a, Frequency of DN (CD4−CD8−), DP (CD4+CD8+), CD4SP (CD4+CD8−) and CD8SP (CD4−CD8+) thymocytes, and absolute numbers of total thymocytes, CD4SP and CD8SP, in 8-wk old WT (n = 5) and Egr2ΔT (n = 4) mice from 2 independent experiments. b, Histograms showing ex vivo EGR2 protein expression in unstimulated and stimulated (PMA+Iono) splenic WT and Egr2ΔT CD4+ T cells; n = 2 independent experiments. c, EGR2 protein expression (left) and Egr2 mRNA abundance (right) in WT and Egr2ΔT TH17 cells (IL-6 + TGF-β1) following PMA+Iono stimulation. Data represent biologically independent replicates from (n = 4) independent experiments. d, Representative contour plots and bar graphs depict the frequency of IL-17A-producing WT (n = 22), Egr1−/− (n = 9), Egr2ΔT (n = 20), Egr3−/− (n = 3), Egr1−/− Egr2ΔT (n = 11), Egr1−/−Egr3−/− (n = 2), Egr2ΔTEgr3−/− (n = 5) and Egr1−/−Egr2ΔT Egr3−/− (n = 3) CD4+ T cells cultured under TH17-cell polarizing conditions as in c. **P < 0.01, *P < 0.05, two-tailed Student’s t test. Mean values ± s.e.m. are shown in a, c, d.
Extended Data Fig. 3 EGR2 is not expressed in CD4+ T cells during colitis.
a, (Left) Combined weight loss curve of Rag2−/− recipients after intraperitoneal injection of naive CD45RBhiCD25− CD4+ T cells isolated from WT (n = 15) or Egr2ΔT (n = 15) mice. Data are presented as percent of original body weight (measured on day 0). Combined data from n = 5 independent experiments. (Right) Bar graph depicts the frequency of EGR2+ CD4+FOXP3− T cells isolated from the colon of healthy (naive) WT (n = 15) mice and Rag2−/− recipients of naïve WT CD4+ T cells at 6 weeks post-transfer (colitis) (n = 9); combined data from 5 (naïve) and 3 (colitis) independent experiments. b, RT-PCR analysis of Egr2 mRNA expression in unstimulated or stimulated (PMA+Iono) TH17(β,6), TH17(β,6,23) and TH17(1,6,23) cells. Egr2 mRNA was normalized to the house-keeping Hprt gene. Data represent biologically independent replicates per condition from n = 2 independent experiments. Mean values ± s.e.m. are reported in a-b.
Extended Data Fig. 4 EGR2 does not control chromatin accessibility in TH17 cells.
a, Bulk RNA-seq Workflow. Naïve 2D2 WT and 2D2 Egr2ΔT CD4+ T cells were activated and differentiated under pathogenic TH17 cell-polarizing conditions (TH17(β,6,23)) for 5 days. Differentiated TH17(β,6,23) cells were reactivated by plate-bound CD3 + CD28 antibodies in the presence of IL-23 for 48 h before adoptive transfer into Tcrb−/− recipients. At the peak of the disease (day 20 post-transfer), donor CD4+ T cells were purified from spleens and CNS of the Tcrb−/− recipient mice using CD4 negative selection for RNA profiling and library was sequenced on a HiSeq4000. Three independent experiments were performed. b, Heatmap illustrating dynamics of chromatin accessibility in 2D2 WT and 2D2 Egr2ΔT TH17(β,6) cells (GSE224960). Data represent biologically independent replicates per condition from n = 3 independent experiments.
Extended Data Fig. 5 EGR2 binds to and transactivates Rorc and Il17a promoters.
a, CUT&Tag Workflow. CUT&Tag sequencing was performed on nuclei isolated from live (propidium iodide− sorted) 2D2 WT and 2D2 Egr2ΔT TH17(β,6) cells at the peak of EGR2 expression (40 h postactivation) using EGR2 antibody or IgG control. b, (Left) Genome browser tracks at Id3 and Ifngr2, showing EGR2 binding in 2D2 WT TH17(β,6) cells. (Right) Genome browser tracks at Il17a and Tbx21 showing no EGR2 binding in 2D2 WT TH17(β,6) cells. c, Evolutionary conserved regions (ECRs) and EGR binding sites within each ECR of Rorc and Il17a genes were analyzed using ECR browser (https://ecrbrowser.dcode.org/) and JASPAR (http://jaspar.genereg.net/). ChIP primers (P) to determine EGR2 binding to predicted EGR binding sites were designed using Primer3 (http://bioinfo.ut.ee/primer3-0.4.0/). d, EGR2 ChIP-PCR analysis of 2D2 TH17(β,6) cells 48 h after activation with plate-bound CD3 + CD28 antibodies, showing EGR2-specific binding to Rorc and Il17a promoters. Crtam intron and Lag3 core promoters were used as positive controls. Ifng promoter and IgG control antibody were used as negative controls. Results are presented as percent of input DNA. Data are shown as mean ± s.e.m. and represent biologically independent replicates from n = 3 independent experiments. e, Firefly luciferase activity (normalized to Renilla) driven by the 2 kb genomic DNA sequences upstream of the start codon (ATG) of Rorc and Il17a was measured in the presence of increasing doses of Egr1- or Egr2-expressing plasmids in HEK293 cells. Data represent technical duplicates and are representative of n = 3 independent experiments. f, Quantitative RT-PCR analysis of ‘pathogenicity-associated’ genes in TH17(β,6) cells transduced with Egr1-RV or Egr2-RV and normalized to empty virus (EV-RV)-transduced control TH17(β,6) cells. RT-PCR analysis was performed on RNA isolated from sorted retrovirally-transduced cells. Data represent biologically independent replicates per condition from n = 2 independent experiments.
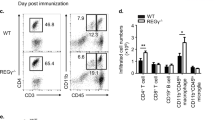
Extended Data Fig. 6 EGR2 is not required for CD4+ T cell activation.
a, CD5 and CD69 protein expression (MFI, frequency) in CD4+ T cells isolated from draining lymph nodes (dLN) and CNS of WT (n = 17) and Egr2ΔT (n = 13) mice following immunization with MOG35-55/CFA and pertussis toxin. ***P < 0.001, *P < 0.05, n.s. = not significant, two-tailed Mann-Whitney U test. b, IL-1R expression in CNS-infiltrating CD4+ T cells from WT (n = 14) and Egr2ΔT (n = 12) mice 14 days postimmunization as in a. n.s. = not significant, two-tailed Student’s t test. c-d, Expression of Ki67 marker of proliferation (c) and Annexin V marker of apoptosis (d) in CD4+ T cells isolated from draining lymph nodes and CNS of WT (n = 14, Ki67; n = 16, AnnexinV) and Egr2ΔT (n = 12, Ki67; n = 16, AnnexinV) mice postimmunization as in a. n.s. = not significant, two-tailed Student’s t test. e, Contour plots depict representative intracellular cytokine staining for IL-17A, IFN-γ and GM-CSF and bar graphs summarize the frequency and the absolute numbers of IL-17A-, IFN-γ- and GM-CSF-producing CD4+ T cells in the draining lymph nodes of WT (n = 10) and Egr2ΔT (n = 8) mice 7 days postimmunization as in a; Data are represented as mean± s.e.m. and are combined from 3 (a-d) and 2 (e) independent experiments.
Extended Data Fig. 7 EGR2 is not required for TH1 cell migration to CNS.
a, Percentage and number of CD4+ T cells in the CNS of Toxoplasma gondii infected WT (n = 10) and Egr2ΔT (n = 8) mice (14 days postinfection). **P < 0.01, n.s. = not significant, two-tailed unpaired Student’s t test. b, Cytokine production by CD4+ T cells in spleen and CNS of WT (n = 10) and Egr2ΔT (n = 8) mice (14 days postinfection). Mean values ± s.e.m. are reported, combined data from 2 independent experiments (a-b). ****P < 0.0001, *P < 0.05, n.s. = not significant, two-tailed Student’s t test.
Extended Data Fig. 8 EGR2 drives regulatory network in pathogenic TH17 cells.
EGR2-regulated module of the TH17 differentiation program controls TH17 cell migration, recruitment of myelomonocytic cells, and the expression of pathogenicity-associated genes.
Supplementary information
Supplementary Fig. 1
General gating strategy for flow cytometry.
Supplementary Table 1
a, All expressed transcripts and DEGs in TH17(β,6/EV), TH17(β,6/Egr2), TH17(β,6,23/EV) and TH17(1,6,23/EV) cells. b, All expressed transcripts and DEGs in 2D2 Egr2ΔT CD4+ T cells compared with 2D2 WT CD4+ T cells from the spleen and CNS of T cell-deficient (Tcrb−/−) mice that received either 2D2 WT or 2D2 Egr2ΔT TH17(β,6,23) cells (20 days posttransfer).
Supplementary Table 2
Hypergeometric testing of DEGs TH17(β,6/EV), TH17(β,6/Egr2), TH17(β,6,23/EV) and TH17(1,6,23/EV) cells against KEGG database; log2 fold change >1 or <−1, BH P < 0.05.
Supplementary Table 3
CUT&Tag: EGR2-bound loci and EGR2-bound genes in TH17(β,6) cells.
Supplementary Table 4
RT–PCR primer sequences.
Supplementary Table 5
ChIP–PCR assay primer sequences.
Rights and permissions
About this article
Cite this article
Gao, Y., Wang, Y., Chauss, D. et al. Transcription factor EGR2 controls homing and pathogenicity of TH17 cells in the central nervous system. Nat Immunol 24, 1331–1344 (2023). https://doi.org/10.1038/s41590-023-01553-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-023-01553-7