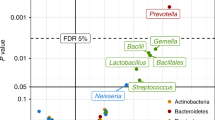
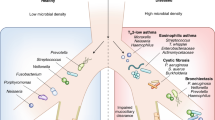
Abstract
Respiratory infections are common in infants and young children. However, the immune system develops and matures as the child grows, thus the effects of infection during this time of dynamic change may have long-term consequences. The infant immune system develops in conjunction with the seeding of the microbiome at the respiratory mucosal surface, at a time that the lungs themselves are maturing. We are now recognizing that any disturbance of this developmental trajectory can have implications for lifelong lung health. Here, we outline our current understanding of the molecular mechanisms underlying relationships between immune and structural cells in the lung with the local microorganisms. We highlight the importance of gaining greater clarity as to what constitutes a healthy respiratory ecosystem and how environmental exposures influencing this network will aid efforts to mitigate harmful effects and restore lung immune health.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Narayanan, M. et al. Alveolarization continues during childhood and adolescence: new evidence from helium-3 magnetic resonance. Am. J. Respir. Crit. Care Med. 185, 186–191 (2012).
Lloyd, C. M. & Saglani, S. Opening the window of immune opportunity: treating childhood asthma. Trends Immunol. 40, 786–798 (2019).
Bosch, A. et al. Maturation of the infant respiratory microbiota, environmental drivers, and health consequences. A prospective cohort study. Am. J. Respir. Crit. Care Med 196, 1582–1590 (2017).
Sandall, J. et al. Short-term and long-term effects of caesarean section on the health of women and children. Lancet 392, 1349–1357 (2018).
Olszak, T. et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science 336, 489–493 (2012).
Russell, S. L. et al. Early-life antibiotic-driven changes in microbiota enhance susceptibility to allergic asthma. EMBO Rep. 13, 440–447 (2012).
Achten, N. B., van Rossum, A. M. C., Bacharier, L. B., Fitzpatrick, A. M. & Hartert, T. V. Long-term respiratory consequences of early-life respiratory viral infections: a pragmatic approach to fundamental questions. J. Allergy Clin. Immunol. Pract. 10, 664–670 (2022).
Shi, T. et al. Risk factors for respiratory syncytial virus associated with acute lower respiratory infection in children under five years: systematic review and meta-analysis. J. Glob. Health 5, 020416 (2015).
Stenberg-Hammar, K. et al. Rhinovirus-specific antibody responses in preschool children with acute wheeze reflect severity of respiratory symptoms. Allergy 71, 1728–1735 (2016).
Oksel, C. et al. Distinguishing wheezing phenotypes from infancy to adolescence. A pooled analysis of five birth cohorts. Ann. Am. Thorac. Soc. 16, 868–876 (2019).
van Meel, E. R. et al. Early-life respiratory tract infections and the risk of school-age lower lung function and asthma: a meta-analysis of 150,000 European children. Eur. Respir. J. 60, 2102395 (2022).
Bose, S., Pascoe, C. & McEvoy, C. Lifetime lung function trajectories and COPD: when the train derails. Lancet Respir. Med. 11, 221–222 (2022).
Zar, H. J. et al. Early-life respiratory syncytial virus lower respiratory tract infection in a South African birth cohort: epidemiology and effect on lung health. Lancet Glob. Health 8, e1316–e1325 (2020).
Belgrave, D. C. M. et al. Lung function trajectories from pre-school age to adulthood and their associations with early life factors: a retrospective analysis of three population-based birth cohort studies. Lancet Respir. Med. 6, 526–534 (2018).
Berry, C. E. et al. A distinct low lung function trajectory from childhood to the fourth decade of life. Am. J. Respir. Crit. Care Med. 194, 607–612 (2016).
Agustí, A., Noell, G., Brugada, J. & Faner, R. Lung function in early adulthood and health in later life: a transgenerational cohort analysis. Lancet Respir. Med. 5, 935–945 (2017).
Olin, A. et al. Stereotypic immune system development in newborn children. Cell 174, 1277–1292 (2018).
Ariyakumar, G. et al. Activation of lymphocytes in healthy neonates within hours of birth. Front. Immunol. 13, 883933 (2022).
Lee, A. H. et al. Dynamic molecular changes during the first week of human life follow a robust developmental trajectory. Nat. Commun. 10, 1092 (2019).
Thome, J. J. et al. Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell 159, 814–828 (2014).
Thome, J. J. et al. Early-life compartmentalization of human T cell differentiation and regulatory function in mucosal and lymphoid tissues. Nat. Med. 22, 72–77 (2016).
Ifrim, D. C. et al. Trained immunity or tolerance: opposing functional programs induced in human monocytes after engagement of various pattern recognition receptors. Clin. Vaccin. Immunol. 21, 534–545 (2014).
Hinks, T. S. et al. Innate and adaptive T cells in asthmatic patients: relationship to severity and disease mechanisms. J. Allergy Clin. Immunol. 136, 323–333 (2015).
van Wilgenburg, B. et al. MAIT cells contribute to protection against lethal influenza infection in vivo. Nat. Commun. 9, 4706 (2018).
Levy, O. & Netea, M. G. Innate immune memory: implications for development of pediatric immunomodulatory agents and adjuvanted vaccines. Pediatr. Res. 75, 184–188 (2014).
de Goffau, M. C. et al. Human placenta has no microbiome but can contain potential pathogens. Nature 572, 329–334 (2019).
Dominguez-Bello, M. G. et al. Partial restoration of the microbiota of cesarean-born infants via vaginal microbial transfer. Nat. Med. 22, 250–253 (2016).
de Steenhuijsen Piters, W. A. A., Binkowska, J. & Bogaert, D. Early life microbiota and respiratory tract infections. Cell Host Microbe 28, 223–232 (2020).
Pattaroni, C. et al. Early-life formation of the microbial and immunological environment of the human airways. Cell Host Microbe 24, 857–865 (2018).
Stearns, J. C. et al. Culture and molecular-based profiles show shifts in bacterial communities of the upper respiratory tract that occur with age. ISME J. 9, 1246–1259 (2015).
Pattaroni, C. et al. Early life inter-kingdom interactions shape the immunological environment of the airways. Microbiome 10, 34 (2022).
Atarashi, K. et al. Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature 500, 232–236 (2013).
Atarashi, K. et al. Induction of colonic regulatory T cells by indigenous Clostridium species. Science 331, 337–341 (2011).
Round, J. L. & Mazmanian, S. K. Inducible Foxp3+ regulatory T cell development by a commensal bacterium of the intestinal microbiota. Proc. Natl Acad. Sci. USA 107, 12204–12209 (2010).
Segal, L. N. et al. Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a TH17 phenotype. Nat. Microbiol. 1, 16031 (2016).
Thorsen, J. et al. Infant airway microbiota and topical immune perturbations in the origins of childhood asthma. Nat. Commun. 10, 5001 (2019).
Natalini, J. G., Singh, S. & Segal, L. N. The dynamic lung microbiome in health and disease. Nat. Rev. Microbiol. 21, 222–235 (2022).
Brunwasser, S. M. et al. Assessing the strength of evidence for a causal effect of respiratory syncytial virus lower respiratory tract infections on subsequent wheezing illness: a systematic review and meta-analysis. Lancet Respir. Med. 8, 795–806 (2020).
Man, W. H. et al. Infant respiratory syncytial virus prophylaxis and nasopharyngeal microbiota until 6 years of life: a subanalysis of the MAKI randomised controlled trial. Lancet Respir. Med. 8, 1022–1031 (2020).
Tang, H. H. F. et al. Developmental patterns in the nasopharyngeal microbiome during infancy are associated with asthma risk. J. Allergy Clin. Immunol. 147, 1683–1691 (2021).
Zuurbier, R. P. et al. Asymptomatic viral presence in early life precedes recurrence of respiratory tract infections. Pediatr. Infect. Dis. J. 42, 59–65 (2023).
Jackson, D. J. & Gern, J. E. Rhinovirus infections and their roles in asthma: etiology and exacerbations. J. Allergy Clin. Immunol. Pract. 10, 673–681 (2022).
Choi, T. et al. Enhanced neutralizing antibody responses to Rhinovirus C and age-dependent patterns of infection. Am. J. Respir. Crit. Care Med. 203, 822–830 (2021).
Calışkan, M. et al. Rhinovirus wheezing illness and genetic risk of childhood-onset asthma. N. Engl. J. Med. 368, 1398–1407 (2013).
Loss, G. J. et al. The early development of wheeze. Environmental determinants and genetic susceptibility at 17q21. Am. J. Respir. Crit. Care Med. 193, 889–897 (2016).
Bønnelykke, K. et al. A genome-wide association study identifies CDHR3 as a susceptibility locus for early childhood asthma with severe exacerbations. Nat. Genet. 46, 51–55 (2014).
Edwards, M. R. et al. Impaired innate interferon induction in severe therapy resistant atopic asthmatic children. Mucosal Immunol. 6, 797–806 (2013).
Altman, M. C. et al. Transcriptome networks identify mechanisms of viral and nonviral asthma exacerbations in children. Nat. Immunol. 20, 637–651 (2019).
Dissanayake, E. et al. Rhinovirus increases Moraxella catarrhalis adhesion to the respiratory epithelium. Front. Cell Infect. Microbiol. 12, 1060748 (2022).
Hasegawa, K. et al. Association of nasopharyngeal microbiota profiles with bronchiolitis severity in infants hospitalised for bronchiolitis. Eur. Respir. J. 48, 1329–1339 (2016).
Raita, Y. et al. Integrated-omics endotyping of infants with rhinovirus bronchiolitis and risk of childhood asthma. J. Allergy Clin. Immunol. 147, 2108–2117 (2021).
Raita, Y. et al. Integrated omics endotyping of infants with respiratory syncytial virus bronchiolitis and risk of childhood asthma. Nat. Commun. 12, 3601 (2021).
Bisgaard, H. et al. Childhood asthma after bacterial colonization of the airway in neonates. N. Engl. J. Med. 357, 1487–1495 (2007).
Robinson, P. F. M. et al. Recurrent severe preschool wheeze: from prespecified diagnostic labels to underlying endotypes. Am. J. Respir. Crit. Care Med. 204, 523–535 (2021).
Ahmed, B. et al. Comparison of the upper and lower airway microbiota in children with chronic lung diseases. PLoS ONE 13, e0201156 (2018).
Stein, M. M. et al. Innate immunity and asthma risk in Amish and Hutterite farm children. N. Engl. J. Med. 375, 411–421 (2016).
Hrusch, C. L. et al. T cell phenotypes are associated with serum IgE levels in Amish and Hutterite children. J. Allergy Clin. Immunol. 144, 1391–1401 (2019).
Illi, S. et al. Protection from childhood asthma and allergy in Alpine farm environments—the GABRIEL Advanced Studies. J. Allergy Clin. Immunol. 129, 1470–1477 (2012).
Depner, M. et al. Maturation of the gut microbiome during the first year of life contributes to the protective farm effect on childhood asthma. Nat. Med. 26, 1766–1775 (2020).
Manolova, V., Flace, A., Jeandet, P., Bessler, W. & Pasquali, C. Biomarkers induced by the immunomodulatory bacterial extract OM-85: unique roles for Peyer’s patches and intestinal epithelial cells. J. Clin. Cell. Immunol. 8, 2 (2017).
Nelson, H. S. The return of the mixed respiratory bacterial vaccine. Allergy Asthma Proc. 43, 501–508 (2022).
Ballarini, S. et al. Can bacterial lysates be useful in prevention of viral respiratory infections in childhood? The results of experimental OM-85 studies. Front. Pediatr. 10, 1051079 (2022).
Roth, M., Pasquali, C., Stolz, D. & Tamm, M. Broncho Vaxom (OM-85) modulates rhinovirus docking proteins on human airway epithelial cells via Erk1/2 mitogen activated protein kinase and cAMP. PLoS ONE 12, e0188010 (2017).
Brandi, P. et al. Trained immunity induction by the inactivated mucosal vaccine MV130 protects against experimental viral respiratory infections. Cell Rep. 38, 110184 (2022).
Chotirmall, S. H. et al. Therapeutic targeting of the respiratory microbiome. Am. J. Respir. Crit. Care Med. 206, 535–544 (2022).
Koenen, M. H., de Steenhuijsen Piters, W. A. A., Bogaert, D. & Verhagen, L. M. The microbiota in respiratory tract infections: from association to intervention. Curr. Opin. Infect. Dis. 35, 215–222 (2022).
Dharmage, S. C. et al. Lifetime spirometry patterns of obstruction and restriction, and their risk factors and outcomes: a prospective cohort study. Lancet Respir. Med. 11, 273–282 (2023).
McEvoy, C. T., Le Souef, P. N. & Martinez, F. D. The role of lung function in determining which children develop asthma. J. Allergy Clin. Immunol. Pract. 11, 677–683 (2023).
Acknowledgements
C.M.L. is supported by the Wellcome Trust (220254/Z/20/Z). S.S. is supported by the National Institute of Health Research Efficacy and Mechanism Evaluation program (17/60/51). Figures were created with BioRender.com.
Author information
Authors and Affiliations
Contributions
C.M.L. and S.S. contributed equally to this Review.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks the anonymous reviewers for their contribution to the peer review of this work. Primary Handling Editor: Jamie D. K. Wilson, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lloyd, C.M., Saglani, S. Early-life respiratory infections and developmental immunity determine lifelong lung health. Nat Immunol 24, 1234–1243 (2023). https://doi.org/10.1038/s41590-023-01550-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-023-01550-w