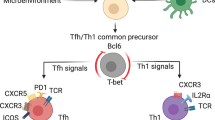
Abstract
Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb), is a global cause of death. Granuloma-associated lymphoid tissue (GrALT) correlates with protection during TB, but the mechanisms of protection are not understood. During TB, the transcription factor IRF4 in T cells but not B cells is required for the generation of the TH1 and TH17 subsets of helper T cells and follicular helper T (TFH)-like cellular responses. A population of IRF4+ T cells coexpress the transcription factor BCL6 during Mtb infection, and deletion of Bcl6 (Bcl6fl/fl) in CD4+ T cells (CD4cre) resulted in reduction of TFH-like cells, impaired localization within GrALT and increased Mtb burden. In contrast, the absence of germinal center B cells, MHC class II expression on B cells, antibody-producing plasma cells or interleukin-10-expressing B cells, did not increase Mtb susceptibility. Indeed, antigen-specific B cells enhance cytokine production and strategically localize TFH-like cells within GrALT via interactions between programmed cell death 1 (PD-1) and its ligand PD-L1 and mediate Mtb control in both mice and macaques.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
Bulk RNA-seq data are available in the NCBI GEO with accession numbers GSE66578 and SRX960261–SRX960267. All macaque bulk RNA-seq reads are available in the NCBI Sequence Read Archive, Bioproject PRJNA523820. scRNA-seq data for B6 mice are available on the GEO under accession number GSE200639. Source data are provided with this paper.
References
World Health Organization. Global Tuberculosis Report (2020); https://www.who.int/teams/global-tuberculosis-programme/tb-reports
Scott, N. R. et al. S100A8/A9 regulates CD11b expression and neutrophil recruitment during chronic tuberculosis. J. Clin. Invest. 130, 3098–3112 (2020).
Slight, S. R. et al. CXCR5+ T helper cells mediate protective immunity against tuberculosis. J. Clin. Invest. 123, 712–726 (2013).
Esaulova, E. et al. The immune landscape in tuberculosis reveals populations linked to disease and latency. Cell Host Microbe 29, 165–178 (2021).
Ahmed, M. et al. Immune correlates of tuberculosis disease and risk translate across species. Sci. Transl. Med. 12, eaay0233 (2020).
Lu, L. L. et al. A functional role for antibodies in tuberculosis. Cell 167, 433–443 (2016).
Lu, L. L. et al. Antibody Fc glycosylation discriminates between latent and active tuberculosis. J. Infect. Dis. 222, 2093–2102 (2020).
Choreño-Parra, J. A. et al. Mycobacterium tuberculosis HN878 infection induces human-like B cell follicles in mice. J. Infect. Dis. 221, 1636–1646 (2020).
Green, A. M., Difazio, R. & Flynn, J. L. IFN-γ from CD4 T cells is essential for host survival and enhances CD8 T cell function during Mycobacterium tuberculosis infection. J. Immunol. 190, 270–277 (2013).
Kaushal, D. et al. Mucosal vaccination with attenuated Mycobacterium tuberculosis induces strong central memory responses and protects against tuberculosis. Nat. Commun. 6, 8533 (2015).
Mittrücker, H. W. et al. Requirement for the transcription factor LSIRF/IRF4 for mature B and T lymphocyte function. Science 275, 540–543 (1997).
Edgar, R., Domrachev, M. & Lash, A. E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210 (2002).
Akter, S. et al. Mycobacterium tuberculosis infection drives a type I IFN signature in lung lymphocytes. Cell Rep. 39, 110983 (2022).
Klein, U. et al. Transcription factor IRF4 controls plasma cell differentiation and class-switch recombination. Nat. Immunol. 7, 773–782 (2006).
De Silva, N. S., Simonetti, G., Heise, N. & Klein, U. The diverse roles of IRF4 in late germinal center B cell differentiation. Immunological Rev. 247, 73–92 (2012).
Mason, D. Y., Jones, M. & Goodnow, C. C. Development and follicular localization of tolerant B lymphocytes in lysozyme/anti-lysozyme IgM/lgD transgenic mice. Int. Immunol. 4, 163–175 (1992).
Liu, H. et al. ADORA1 inhibition promotes tumor immune evasion by regulating the ATF3–PD-L1 axis. Cancer Cell 37, 324–339 (2020).
Tan, C. et al. NR4A nuclear receptors restrain B cell responses to antigen when second signals are absent or limiting. Nat. Immunol. 21, 1267–1279 (2020).
Reif, K. et al. Balanced responsiveness to chemoattractants from adjacent zones determines B cell position. Nature 416, 94–99 (2002).
Nam, S. & Lim, J. S. Essential role of interferon regulatory factor 4 (IRF4) in immune cell development. Arch. Pharm. Res. 39, 1548–1555 (2016).
Man, K. et al. Transcription factor IRF4 promotes CD8+ T cell exhaustion and limits the development of memory-like T cells during chronic infection. Immunity 47, 1129–1141 (2017).
Wu, J. et al. Ablation of transcription factor IRF4 promotes transplant acceptance by driving allogenic CD4+ T cell dysfunction. Immunity 47, 1114–1128 (2017).
Jayaraman, P. et al. TIM3 mediates T cell exhaustion during Mycobacterium tuberculosis infection. PLoS Pathog. 12, e1005490 (2016).
Man, K. et al. The transcription factor IRF4 is essential for TCR affinity-mediated metabolic programming and clonal expansion of T cells. Nat. Immunol. 14, 1155–1165 (2013).
Caruso, A. M. et al. Mice deficient in CD4 T cells have only transiently diminished levels of IFN-γ, yet succumb to tuberculosis. J. Immunol. 162, 5407–5416 (1999).
Johnston, R. J. et al. Bcl6 and Blimp-1 are reciprocal and antagonistic regulators of T follicular helper cell differentiation. Science 325, 1006–1010 (2009).
du Plessis, W. J., Keyser, A., Walzl, G. & Loxton, A. G. Phenotypic analysis of peripheral B cell populations during Mycobacterium tuberculosis infection and disease. J. Inflamm. 13, 23 (2016).
Zimmermann, N. et al. Human isotype-dependent inhibitory antibody responses against Mycobacterium tuberculosis. EMBO Mol. Med. 8, 1325–1339 (2016).
Ordoñez, C. et al. Both B-1a and B-1b cells exposed to Mycobacterium tuberculosis lipids differentiate into IgM antibody-secreting cells. Immunology 154, 613–623 (2018).
Rao, M. et al. B in TB: B cells as mediators of clinically relevant immune responses in tuberculosis. Clin. Infect. Dis. 61, S225–S234 (2015).
Allie, S. R. et al. The establishment of resident memory B cells in the lung requires local antigen encounter. Nat. Immunol. 20, 97–108 (2019).
Giles, J. R., Kashgarian, M., Koni, P. A. & Shlomchik, M. J. B cell-specific MHC class II deletion reveals multiple nonredundant roles for B cell antigen presentation in murine lupus. J. Immunol. 195, 2571–2579 (2015).
Rodriguez-Pinto, D. & Moreno, J. B cells can prime naive CD4+ T cells in vivo in the absence of other professional antigen-presenting cells in a CD154-CD40-dependent manner. Eur. J. Immunol. 35, 1097–1105 (2005).
Redford, P. S., Murray, P. J. & O’Garra, A. The role of IL-10 in immune regulation during M. tuberculosis infection. Mucosal Immunol. 4, 261–270 (2011).
Nus, M. et al. Marginal zone B cells control the response of follicular helper T cells to a high-cholesterol diet. Nat. Med. 23, 601–610 (2017).
Shi, J. et al. PD-1 controls follicular T helper cell positioning and function. Immunity 49, 264–274 (2018).
Reiley, W. W. et al. Distinct functions of antigen-specific CD4 T cells during murine Mycobacterium tuberculosis infection. Proc. Natl Acad. Sci. USA 107, 19408–19413 (2010).
Barber, D. L., Mayer-Barber, K. D., Feng, C. G., Sharpe, A. H. & Sher, A. CD4 T cells promote rather than control tuberculosis in the absence of PD-1-mediated inhibition. J. Immunol. 186, 1598–1607 (2011).
Kauffman, K. D. et al. PD-1 blockade exacerbates Mycobacterium tuberculosis infection in rhesus macaques. Sci. Immunol. 6, eabf3861 (2021).
Sakai, S. et al. CD4 T cell-derived IFN-γ plays a minimal role in control of pulmonary mycobacterium tuberculosis infection and must be actively repressed by PD-1 to prevent lethal disease. PLoS Pathog. 12, e1005667 (2016).
Das, S. et al. Lung epithelial signaling mediates early vaccine-induced CD4+ T cell activation and Mycobacterium tuberculosis control. mBio 12, e01468-21 (2021).
Ahmed, S. et al. Host-directed therapy as a novel treatment strategy to overcome tuberculosis: targeting immune modulation. Antibiotics 9, 21 (2020).
Phuah, J. et al. Effects of B cell depletion on early Mycobacterium tuberculosis infection in cynomolgus macaques. Infect. Immun. 84, 1301–1311 (2016).
Deenick, E. K. et al. Follicular helper T cell differentiation requires continuous antigen presentation that is independent of unique B cell signaling. Immunity 33, 241–253 (2010).
Haynes, W. A. et al. Empowering multi-cohort gene expression analysis to increase reproducibility. Pac. Symp. Biocomput. 22, 144–153 (2016).
Khader, S. A. et al. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 8, 369–377 (2007).
Zak, D. E. et al. ACS and GC6-74 cohort study groups. A blood RNA signature for tuberculosis disease risk: a prospective cohort study. Lancet 387, 2312–2322 (2016).
Mahomed, H. et al. Predictive factors for latent tuberculosis infection among adolescents in a high-burden area in South Africa. Int J. Tuberc. Lung Dis. 15, 331–336 (2011).
Zerbino, D. R. et al. Ensembl 2018. Nucleic Acids Res. 46, D754–D761 (2018).
Kim, D., Langmead, B. & Salzberg, S. L. HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360 (2015).
Anders, S. & Huber, W. Differential expression analysis for sequence count data. Genome Biol. 11, R106 (2010).
Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 57, 289–300 (1995).
Acknowledgements
This work was supported by Washington University in St. Louis and University of Chicago; NIH grants HL105427 and AI111914 to S.A.K.; AI123780 to S.A.K., D.K., T.S. and M.M.; AI134236 to S.A.K. and D.K.; R01AI134240, R01AI138587, P51OD011133, P51OD011104, U42OD010442, S10OD028732 and C06OD030079 to D.K.; and NIH grant AR069655 (Center for Musculoskeletal Research, University of Rochester). We thank M. Veschak, M. D. Dunlap and S. Thirunavukkarasu of Washington University of St. Louis, Department of Molecular Microbiology, and A. Seeger and L. Reese of University of Rochester, Department of Surgical Pathology for technical support and assistance.
Author information
Authors and Affiliations
Contributions
R.V.S., A.G., D.K. and S.A.K. designed experiments, analyzed data, constructed figures and wrote the manuscript. D.K. and S.A.K. initiated the study and supervised all aspects. R.V.S. and A.G. performed the experiments. T.W.F., L.L., J.A.C.-P., S.K.M., B.A.R., S.A., S.D., M.A., M.L.G.-H., D.K.S., E.E., M.N.A., J.G., S.M., J.Z., M.M., T.J.S. and J.R.-M. performed specific experiments, contributed resources and/or data analysis. M.M., D.K., T.J.S. and S.A.K provided funding. All authors reviewed, edited and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Immunology thanks Andreas Kupz and the other, anonymous, reviewers for their contribution to the peer review of this work. Primary Handling Editor: L. A. Dempsey, in collaboration with the Nature Immunology team.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Irf4 expression by CD19+ B cells is required for accumulation of FO B cells in the lung.
Irf4fl/fl, CD4creIrf4fl/fl, and CD19creIrf4fl/fl mice (n = 5–10 mice/group) were infected with Mtb HN878 and euthanized at 25 and 50 dpi. (a) MHC-IIhi AMs, (b) neutrophils, (c) total B cells, (d) GC B cells, and (e) FO B cells were determined by flow cytometry. Sera collected from mice (50 dpi; dilution shown 1:30) was analyzed via ELISA for levels of (f) IgG2a and IgG2b specific for Mtb antigen CFP-10. Data represent mean + SD, analysis was performed using one-way ANOVA with Tukey’s multiple comparison test (a, c, d, f), and Kruskal-Wallis ANOVA with Dunn’s multiple comparison test (b, e). *, p ≤ 0.05; **, p ≤ 0.005; ***, p ≤ 0.0005.
Extended Data Fig. 2 Bcl6 expression by CD19+ B cells is required for optimal infiltration by B cell subsets in the Mtb-infected lung.
scRNA-seq of cells isolated from the lungs of C57BL/6 mice, either uninfected (n = 2 mice), or Mtb-infected at 50 dpi (n = 3 mice) or 100 dpi (n = 3 mice). (a) Swarm plot showing expression of Bcl6, a downstream target of Irf4, in CD4+, CD8+ T and B cells. Bcl6fl/fl, CD4creBcl6fl/fl, and CD19creBcl6fl/fl mice (n = 5–9 mice/group) were infected with Mtb HN878, euthanized at 50 and 100 dpi, and lungs analyzed by flow cytometry for (b) MHC-IIhi AMs, (c) neutrophils, (d) total B cells, (e) GC B cells, and (f) FO B cells. (g) IgG2a and IgG2b antibody specific against Mtb antigen CFP-10 in peripheral blood serum (diluted 1:30) collected at 50 dpi. (h) Tfh-like cells in the lungs of Bcl6fl/fl vs CD19creBcl6fl/fl mice. Data represent mean + SD, analysis was performed using Kruskal-Wallis ANOVA with Dunn’s multiple comparison test (b), one-way ANOVA with Tukey’s multiple comparison test (c to g), and two-sided unpaired t-test (h). *, p ≤ 0.05; **, p ≤ 0.005; ***, p ≤ 0.0005.
Extended Data Fig. 3 Blimp1 deficiency in B cells impairs antibody production in mice.
iABfl/fl, and CD19creiABfl/fl mice (n = 6–8 mice/group) were infected with Mtb HN878, euthanized at 50 and 100 dpi, and lungs analyzed by flow cytometry for (a) Tfh-like cells. Blimp1fl/fl, and CD19creBlimp1fl/fl mice (n = 6–8 mice/group) were infected with Mtb HN878, euthanized at 50 and 100 dpi. Lungs analyzed by flow cytometry for (b) Tfh-like cells. (c) Immunoglobulin specificity against Mtb antigen CFP-10 in peripheral blood serum (diluted 1:30) collected at 50 dpi were detected by ELISA. Il10fl/fl, and CD19creIl10fl/fl mice (n = 6–8 mice/group) were infected with Mtb HN878 and euthanized at 50 and 100 dpi. (d) Tfh-like cells were enumerated by flow cytometry. Data represent mean + SD and statistical analysis was performed with two-sided unpaired t-test. *, p ≤ 0.05; **, p ≤ 0.005; ***, p ≤ 0.0005.
Extended Data Fig. 4 IghelMD4 mice show reduced Mtb-specific antibody production.
B6 and IghelMD4 mice (n = 5–10 mice/group) were infected with Mtb HN878, euthanized at 50, 75 and 100 dpi and organs were collected and processed. (a) CD44hiCD4+ T cells, (b) IFNγ-producing CD4+ T cells, (c) TNFα-producing CD4+ T cells, (d) total B cells, (e) GC B cells, and (f) FO B cells were determined by flow cytometry. (g) Immunoglobulin specificity against Mtb antigen CFP-10 in peripheral blood serum (diluted 1:15) collected at 100 dpi. Data is mean + SD, analysis was performed using two-sided unpaired t-test (a to f), and two-sided Mann-Whitney U-test (g). *, p ≤ 0.05; **, p ≤ 0.005.
Extended Data Fig. 5 B cell depletion in Mtb-infected mice compromises the accumulation of Tfh-like cells in the lung.
C57BL/6 mice (n = 3–5 mice/group) were infected with Mtb, and infection established over 50 days. At 50 dpi, mice either received B cell depleting antibodies (α-B220 and α-CD19) or isotype via the intratracheal route every 3–4 days, over a 25 days period. Mice were euthanized at 75 dpi, and the lungs collected and processed. (a) Experimental scheme. Flow cytometry was used to enumerate (b) CD19+ B220hi B cells, and (c) immunoglobulin specificity against Mtb antigen CFP-10 in peripheral blood serum (diluted 1:30) was determined via ELISA. (d) Bacterial burden was determined in the lungs of Mtb-infected mice receiving either isotype or B cell-depleting antibodies. Formalin-fixed lung lobes were cut and stained to analyze (e) inflammation (average area) and (f) average area of GrALT. Flow cytometry was used to enumerate (g) activated CD4+ T cells, (h) activated CD4+ Tfh-like cells, (i) IL17+ CD4+ Tfh-like cells and (j) IFNγ+ CD4+ Tfh-like cells in the lungs. Data represents mean ± SD, and statistical analyses performed using two-sided unpaired t-test. *, p ≤ 0.05; **, p ≤ 0.005.
Extended Data Fig. 6 Strategic positioning of PD1+ Tfh-like cells within GrALT structures mediates Mtb control.
(a) Average area of GrALT collated from all mouse models (n = 3–10 mice/group) used in studies at 50 dpi and 128 dpi. (b) Representative images from FFPE lung sections stained with CD3 (red), PD-1 (green) and CD45R/B220 (white) in indicated models at 50 or 128 dpi. Data represent mean + SD and statistical analysis was performed using one-way ANOVA with Tukey’s multiple comparison test (3 groups), and two-tailed unpaired t-test (2 groups). *, p ≤ 0.05; **, p ≤ 0.005.
Extended Data Fig. 7 Mtb 16sRNA is located outside GrALT areas and Pd1+ T cells are critical to control Mtb in mice.
FFPE lungs sections from Mtb-infected B6 and gene deficient mice were stained for the presence of Mtb 16S rRNA via in situ hybridization (ISH) at (a) 30, 50, and 75 dpi. FFPE lungs sections from Rag1KO Mtb-infected mice that either received WT T cells:WT B cells or Pd1KO T cells:WT B cells, were stained for (b) the presence of Mtb 16S rRNA via in situ hybridization (ISH) at 30 dpi, (c) presence of GrALT via immunofluorescence; CD3 (red); B220 (white); PD-1 (green).
Extended Data Fig. 8 Compromised accumulation of Tfh-like cells in macaques and mice lungs dampens vaccine mediated protection against Mtb.
Macaques were aerosol exposed to MtbΔsigH prior to challenge with virulent Mtb CDC1551 and received either CD20 depleting (n = 4 NHPs) or IgG isotype control antibodies (n = 2 NHPs). Clinical samples were collected throughout the study and at necropsy. (a) FACS profiles for detection of B cells in PBMCs (left panel), BAL (middle panel), and lung (right panels) in CD20-depleted (upper panels) and control macaques (lower panels). (b) The percentage of total peripheral blood B cells (flow cytometry) and (c) Levels of C-reactive protein (CRP) were determined from samples collected throughout the study. (d) FACS profile for detection of T cell subsets in macaque lungs. (e) ESAT6+ CD4+ T cells producing IFNγ, IL2, IL17 and TNFα in lung tissue were enumerated by flow cytometry at the final end-point. (f) CFP-10 specific antibody levels in serum (diluted 1:15) collected at indicated time points. Data represent mean + SD and statistical analysis was performed with two-sided Mann-Whitney U-test. ***, p≤0.0005. Bcl6fl/fl and CD4creBcl6fl/fl mice (n = 4 to 7 mice/group) were mucosally vaccinated with BCG and rested for thirty days following which mice were infected with Mtb HN878. Mice were euthanized at 20 dpi. (g) Experimental scheme. Lungs were collected and bacterial burden was determined in (h) Bcl6fl/fl and CD4creBcl6fl/fl mice. Data represent mean + SD and statistical analysis was performed with two-sided unpaired t-test. **, p ≤ 0.005.
Extended Data Fig. 9 Gating strategy for B cells and T cells flow-cytometric analysis.
Representative FACS plots with gates defining (a) total B cells, GC B cells, and FO B cells, and (b) CD4 T cells, cytokine producing Th1 cells, Tfh-like cells and cytokine producing Tfh-like cells.
Extended Data Fig. 10 Graphical abstract: Mtb specific B cells and Tfh-like cells interact to mediate protection against Mtb.
B cell effector functions including formation of GC B cells, IL-10 production and antibody/plasma cells have non-essential roles during in vivo Mtb infection. However, Mtb antigen-specific B cells interact with Tfh-like cells likely through PDL1-PD1 to drive differentiation and effective localization of cytokine-producing Tfh-like cells within GrALT to mediate Mtb control (left). Key transcription factors Irf4 and Bcl6 (blue text) are essential for differentiation of Tfh-like cells from naive CD4+ T cells. PD1-PDL1 (blue text) receptor-ligand axis likely drive Tfh-like cell localization and homing into GrALT but this interaction is independent of TCR-MHCII interaction, IL-10 production or antibody response (black text). Thus, Tfh-like cells and B cell interactions play critical roles in mediating Mtb control within GrALT.
Supplementary information
Supplementary Table 1
Ten cohort studies and their accession numbers, used for IRF4 expression analysis.
Supplementary Table 2
Top transcription factors coexpressed with Irf4 upon Mtb infection in mouse.
Source data
Source Data
Statistical source data for main figures.
Source Data
Statistical source data for extended data figures.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Swanson, R.V., Gupta, A., Foreman, T.W. et al. Antigen-specific B cells direct T follicular-like helper cells into lymphoid follicles to mediate Mycobacterium tuberculosis control. Nat Immunol 24, 855–868 (2023). https://doi.org/10.1038/s41590-023-01476-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-023-01476-3
This article is cited by
-
B cell heterogeneity in human tuberculosis highlights compartment-specific phenotype and functional roles
Communications Biology (2024)
-
Glutamine metabolism inhibition has dual immunomodulatory and antibacterial activities against Mycobacterium tuberculosis
Nature Communications (2023)