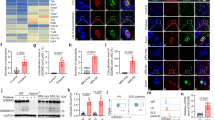
Abstract
The linkage between neutrophil death and the development of autoimmunity has not been thoroughly explored. Here, we show that neutrophils from either lupus-prone mice or patients with systemic lupus erythematosus (SLE) undergo ferroptosis. Mechanistically, autoantibodies and interferon-α present in the serum induce neutrophil ferroptosis through enhanced binding of the transcriptional repressor CREMα to the glutathione peroxidase 4 (Gpx4, the key ferroptosis regulator) promoter, which leads to suppressed expression of Gpx4 and subsequent elevation of lipid-reactive oxygen species. Moreover, the findings that mice with neutrophil-specific Gpx4 haploinsufficiency recapitulate key clinical features of human SLE, including autoantibodies, neutropenia, skin lesions and proteinuria, and that the treatment with a specific ferroptosis inhibitor significantly ameliorates disease severity in lupus-prone mice reveal the role of neutrophil ferroptosis in lupus pathogenesis. Together, our data demonstrate that neutrophil ferroptosis is an important driver of neutropenia in SLE and heavily contributes to disease manifestations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All raw source data for all experiments included in this study are provided. RNA sequencing data that support the findings of this study have been deposited with the Gene Expression Omnibus (GEO) repository under accession number GSE153781. Correspondence and requests for materials should be addressed to zxpumch2003@sina.com. Source data are provided with this paper.
References
Tsokos, G. C. Systemic lupus erythematosus. N. Engl. J. Med. 365, 2110–2121 (2011).
Lisnevskaia, L., Murphy, G. & Isenberg, D. Systemic lupus erythematosus. Lancet 384, 1878–1888 (2014).
Garcia-Romo, G. S. et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 3, 73ra20 (2011).
Bosch, X. Systemic lupus erythematosus and the neutrophil. N. Engl. J. Med. 365, 758–760 (2011).
Dixon, S. J. et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell 149, 1060–1072 (2012).
Yang, W. S. et al. Regulation of ferroptotic cancer cell death by GPX4. Cell 156, 317–331 (2014).
Alim, I. et al. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell 177, 1262–1279.e25 (2019).
Ingold, I. et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell 172, 409–422.e421 (2018).
Friedmann Angeli, J. P. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191 (2014).
Do Van, B. et al. Ferroptosis, a newly characterized form of cell death in Parkinson’s disease that is regulated by PKC. Neurobiol. Dis. 94, 169–178 (2016).
Matsushita, M. et al. T cell lipid peroxidation induces ferroptosis and prevents immunity to infection. J. Exp. Med. 212, 555–568 (2015).
van Vollenhoven, R. F. et al. Efficacy and safety of ustekinumab, an IL-12 and IL-23 inhibitor, in patients with active systemic lupus erythematosus: results of a multicentre, double-blind, phase 2, randomised, controlled study. Lancet 392, 1330–1339 (2018).
ter Borg, E. J., Horst, G., Hummel, E. J., Limburg, P. C. & Kallenberg, C. G. Measurement of increases in anti-double-stranded DNA antibody levels as a predictor of disease exacerbation in systemic lupus erythematosus. A long-term, prospective study. Arthritis Rheum. 33, 634–643 (1990).
Pisetsky, D. S. Anti-DNA antibodies—quintessential biomarkers of SLE. Nat. Rev. Rheumatol. 12, 102–110 (2016).
Lood, C. et al. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 22, 146–153 (2016).
Papayannopoulos, V., Metzler, K. D., Hakkim, A. & Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 191, 677–691 (2010).
Stockwell, B. R. et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171, 273–285 (2017).
Friedmann Angeli, J. P. et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat. Cell Biol. 16, 1180–1191 (2014).
Sun, Y., Zheng, Y., Wang, C. & Liu, Y. Glutathione depletion induces ferroptosis, autophagy, and premature cell senescence in retinal pigment epithelial cells. Cell Death Dis. 9, 753 (2018).
Cohen, P. L. & Eisenberg, R. A. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu. Rev. Immunol. 9, 243–269 (1991).
Dubois, E. L., Horowitz, R. E., Demopoulos, H. B. & Teplitz, R. NZB/NZW mice as a model of systemic lupus erythematosus. JAMA 195, 285–289 (1966).
Ginzler, E. M. et al. Mycophenolate mofetil or intravenous cyclophosphamide for lupus nephritis. N. Engl. J. Med. 353, 2219–2228 (2005).
Alim, I. et al. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell 177, 1262–1279.e25 (2019).
Ingold, I. et al. Selenium utilization by GPX4 is required to prevent hydroperoxide-induced ferroptosis. Cell 172, 409–422.e21 (2018).
Clark, S. R. et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 13, 463–469 (2007).
Speckmann, B. et al. Induction of glutathione peroxidase 4 expression during enterocytic cell differentiation. J. Biol. Chem. 286, 10764–10772 (2011).
De Cesare, D., Fimia, G. M. & Sassone-Corsi, P. Signaling routes to CREM and CREB: plasticity in transcriptional activation. Trends Biochem. Sci. 24, 281–285 (1999).
Hedrich, C. M. et al. cAMP response element modulator α controls IL2 and IL17A expression during CD4 lineage commitment and subset distribution in lupus. Proc. Natl Acad. Sci. USA 109, 16606–16611 (2012).
Hedrich, C. M., Rauen, T., Kis-Toth, K., Kyttaris, V. C. & Tsokos, G. C. cAMP-responsive element modulator α (CREMα) suppresses IL-17F protein expression in T lymphocytes from patients with systemic lupus erythematosus (SLE). J. Biol. Chem. 287, 4715–4725 (2012).
Juang, Y. T. et al. Systemic lupus erythematosus serum IgG increases CREM binding to the IL-2 promoter and suppresses IL-2 production through CaMKIV. J. Clin. Invest. 115, 996–1005 (2005).
Triantafyllopoulou, A. et al. Proliferative lesions and metalloproteinase activity in murine lupus nephritis mediated by type I interferons and macrophages. Proc. Natl Acad. Sci. USA 107, 3012–3017 (2010).
Zhuang, H. et al. Toll-like receptor 7-stimulated tumor necrosis factor α causes bone marrow damage in systemic lupus erythematosus. Arthritis Rheumatol. 66, 140–151 (2014).
Kolb, J. P., Oguin, T. H. 3rd, Oberst, A. & Martinez, J. Programmed cell death and inflammation: winter is coming. Trends Immunol. 38, 705–718 (2017).
Tsokos, G. C., Lo, M. S., Costa Reis, P. & Sullivan, K. E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 12, 716–730 (2016).
Ren, Y. et al. Increased apoptotic neutrophils and macrophages and impaired macrophage phagocytic clearance of apoptotic neutrophils in systemic lupus erythematosus. Arthritis Rheum. 48, 2888–2897 (2003).
Shi, J. et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature 526, 660–665 (2015).
Sun, L. et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 148, 213–227 (2012).
Stockwell, B. R. et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell 171, 273–285 (2017).
Maeshima, E., Liang, X. M., Goda, M., Otani, H. & Mune, M. The efficacy of vitamin E against oxidative damage and autoantibody production in systemic lupus erythematosus: a preliminary study. Clin. Rheumatol. 26, 401–404 (2007).
Legrand, A. J., Konstantinou, M., Goode, E. F. & Meier, P. The diversification of cell death and immunity: memento mori. Mol. Cell 76, 232–242 (2019).
Martin-Sanchez, D. et al. Ferroptosis, but not necroptosis, is important in nephrotoxic folic acid-induced AKI. J. Am. Soc. Nephrol. 28, 218–229 (2017).
Hu, C. L. et al. Reduced expression of the ferroptosis inhibitor glutathione peroxidase-4 in multiple sclerosis and experimental autoimmune encephalomyelitis. J. Neurochem. 148, 426–439 (2019).
Hochberg, M. C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 40, 1725 (1997).
Uribe, A. G. et al. The Systemic Lupus Activity Measure-Revised, the Mexican Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), and a modified SLEDAI-2K are adequate instruments to measure disease activity in systemic lupus erythematosus. J. Rheumatol. 31, 1934–1940 (2004).
Kay, J. & Upchurch, K. S. ACR/EULAR 2010 rheumatoid arthritis classification criteria. Rheumatology (Oxford) 51, vi5–vi9 (2012).
van der Linden, S., Valkenburg, H. A. & Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 27, 361–368 (1984).
International Team for the Revision of the International Criteria for Behçet’s Disease (ITR-ICBD). The International Criteria for Behçet’s Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J. Eur. Acad. Dermatol. Venereol. 28, 338-347 (2014).
Knight, J. S. et al. Peptidylarginine deiminase inhibition disrupts NET formation and protects against kidney, skin and vascular disease in lupus-prone MRL/lpr mice. Ann. Rheum. Dis. 74, 2199–2206 (2015).
Papayannopoulos, V., Metzler, K. D., Hakkim, A. & Zychlinsky, A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 191, 677–691 (2010).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the \(2^{{-\Delta \Delta}{\rm {C}_T}}\) method. Methods 25, 402–408 (2001).
Acknowledgements
This study was supported by National Natural Science Foundation of China grants no. 81788101 (to X.Z.) and no. 81630044 (to X.Z.); Chinese Academy of Medical Science Innovation Fund for Medical Sciences grants no. CIFMS2016-12M-1-003 (to X.Z.), no. 2017-12M-1-008 (to X.Z.), no. 2017-I2M-3-011 (to X.Z.) and no. 2016-12M-1-008 (to X.Z.); Capital’s Funds for Health Improvement and Research (grant no. 2020-2-4019) (to X.Z.); and NIH grants no. R01AR064350 (to G.C.T) and no. R37AI049954 (to G.C.T). We thank A. Davidson at Feinstein Institutes for Medical Research for providing the adenovirus IFN-α. We thank the staff of the Rheumatology and Immunology Laboratory and Medical Scientific Research Center in Peking Union Medical College Hospital (PUMCH) for providing experimental equipment. We thank the doctors at PUMCH, Anyang District Hospital of Henan Province, Xiangya Hospital, Huaian No.1 People’s Hospital and People’s Hospital of Xinjiang Uygur Autonomous Region for patient recruitment.
Author information
Authors and Affiliations
Contributions
X.Z. and P.L. conceived the project and designed the experiments. P.L., M.J., K.L. and H.L. performed most of the experiments with help from X.X., Y.X. and S.K. Y.Z. and H.L. contributed to discussions. P.L., H.L. and P.E.L. wrote the manuscript. G.C.T. and X.Z. supervised work and acquired funding.
Corresponding authors
Ethics declarations
Competing interests
P.E.L. is an employee of AMPEL but has no competing interests with the content of this manuscript. All the authors declare no competing interests.
Additional information
Peer review information Nature Immunology thanks Marcus Conrad, Christian Lood and Chandra Mohan for their contribution to the peer review of this work. Peer reviewer reports are available. L. A. Dempsey was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 IgG and IFNα but not CXCL11 or IL12/23 p40 present in SLE sera contribute to neutropenia.
a-b. Flow cytometry quantification of cell viability of neutrophils (a: n = 3; b: n = 9) in vitro cultured with (a) 5%, 10%, or 20% SLE serum for 6, 16, 24 hours respectively, or with (b) 20 % HC, SLE or RA serum respectively for 16 hours. c. Detection of the inflammatory factors in the sera from RA (n = 16), BD (n = 20) and AS (n = 18) patients vs. HCs (n = 19). d-e. Flow cytometry quantification of cell viability and lipid ROS of HC neutrophils (d: n = 7; e: n = 7 for anti-CXCL11 or n = 4 for Ustekinumab) cultured in vitro with 20% SLE serum supplemented with anti-CXCL11 (0.1, 1, 5 µg ml-1) or Ustekinumab (0.1, 1, 10 µg ml-1) for 16 hours. f-g. The proportion of anti-dsDNA in total IgG correlated with SLE neutrophil counts and SLEDAI scores (n = 63). h. Western blot validation of purified IgG from serum and of serum with IgG depletion. i. Flow cytometry quantification of cell viability of neutrophils (n = 5) cultured in vitro with serum in the presence or absence of anti-IFNAR (10 µg ml-1), or IgG depletion, for 16 hours. j-k. Serum IgG was purified or depleted by Protein A/G. (j) Ponceau S staining (upper panel) and western blot (lower panel) detection of purified IgG and of serum with depleted IgG. (k) Flow cytometry quantification of cell viability of neutrophils (n = 4) with HC serum in the presence of HC or SLE IgG at different concentrations (1.2, 2.4, 3.6 g L-1), or SLE serum with/without IgG depletion, for 16 hours. Data are shown as mean ± SD. *p < 0.05, **p < 0.01, ns p > 0.05. Two-tailed paired or unpaired Student’s t-test was applied.
Extended Data Fig. 2 Ferroptosis is restricted in neutrophils but not other cells in SLE and this could be reverted by addition of Ferroptosis specific inhibitors.
a. Flow cytometry quantification of cell viability of HC lymphocytes, monocytes, and neutrophils cultured with 20% HC or SLE serum for 16 hours and the proportion of apoptotic (Annexin V + 7AAD-), necrotic (Annexin V + 7AAD + ) and live (Annexin V- 7AAD-) cells in each subset was analyzed (n = 6). b. HC neutrophils were cultured with 20% HC or SLE serum, and lipid-ROS productions at different time points were detected (n = 3). c. Dot plots show cell viability analyzed by lactate dehydrogenase (LDH) release. HC neutrophils (n = 9) were cultured with 20% HC serum supplemented with RSL-3 (10 μM) or SLE serum supplemented with LPX-1 (1 μM) for 16 hours before analysis. d-e. Dot plots show flow cytometry quantification of the percentage of apoptotic, necrotic, and live cells. HC neutrophils (n = 5) were cultured with 20% HC or SLE serum in the presence or absence of LPX-1 (1 μM) for 16 hours before analysis. f-g. HC B cells (n = 6) were cultured in 20% HC or SLE serum supplemented with LPX-1 for 72 hours, and plasmacytoid dendritic cells (pDC) (n = 3) were cultured for 24 hours, the level of IgG was assessed by ELISA and type I IFNs by flow cytometry individually. h-i. Dot plots show cell viability and lipid-ROS in HC neutrophils (n = 7) cultured with 20% HC or SLE serum supplemented with ß-ME (10/50 μM) for 16 hours. Data are shown as mean ± SD. ns p > 0.05. Two-tailed paired Student’s t-test was applied.
Extended Data Fig. 3 The cooperative effects between IFNα and SLE IgG on cell death.
a-c. HC neutrophils were cultured in the presence of HC or SLE serum with or without the addition of Cl-amidine (Cl) (peptidyl arginine deiminase 4 (PAD4) inhibitor, 100 μM), or LPX-1 (1 μM) for 4 or 16 hours and NETs were assessed in SYTOX Green+ cells based on morphology (n = 6). Neutrophils with DNA area greater than 400um2 were considered as NETs. Dot plots show the percentage of cells forming NETs in all dead neutrophils from the indicated group. d-e. Representative fluorescent images and related quantification of NETosis. HC neutrophils (n = 6) were stimulated by PMA (50 nM) with or without LPX-1(1 μM) for 4 hours. f-i. HC neutrophils were cultured with SLE IgG (3.6 g L-1) and/or IFN-α (10^5 U ml-1) for 4 or 16 hours and cells were stained with SYTOX Green for the detection of NETs. Dot plots show the immunofluorescence microscope quantification of NETosis in total dead neutrophils from the indicated group (4 h: n = 6; 16 h: n = 3). The scale bar represents 50 μm. Data are shown as mean ± SD. ns p > 0.05. Two-tailed paired Student’s t-test was applied.
Extended Data Fig. 4 The ferroptosis inhibitor ameliorates lupus progression with much better therapeutic effect compared to the NETosis inhibitor.
a-i. MRL/lpr mice (n = 6) were treated with DMSO (0.1 ml 10%), LPX-1(10 mg/kg) or CTX (20 mg/kg) every other day at week 12 for 6 weeks, DMSO (0.1 ml 10%) was applied to sex-matched MRL/Mpj mice (n = 5) as control. Mice were euthanized at 18 weeks of age for analysis. (a) Flow cytometry quantification of lipid ROS. (b) Representative immunofluorescent images of glomeruli stained with IgG (red), IgM (yellow), C1q (green), and DAPI (blue). (c-e) Flow cytometry quantification of plasma inflammatory factors and (f-i) plasma IgG. j-o. MRL/lpr mice (DMSO, LPX-1: n = 3; Cl, Cl+LPX-1: n = 4) were treated with DMSO, Cl, LPX-1, or Cl combined with LPX-1 every other day for 3 weeks starting at the age of week 12. Mice were euthanized at 15 weeks of age for analysis. (j-k) Representative images and related quantification of axillary spleens and lymph nodes. (l) Western blot analysis of cit-H3 in circulating neutrophils from mice subjected to the indicated treatment. (m) Dot plots show the ELISA assessment of serum complement 3. (n) Dot plots show the ELISA assessment of serum anti-dsDNA antibodies titers. (o) Dot plots shows the Bicinchoninic acid (BCA) assay of urine proteins. The scale bar represents 50 μm. Data are shown as mean ± SD. ns p > 0.05. Two-tailed unpaired Student’s t-test was applied.
Extended Data Fig. 5 The expression of cystine transporter SLC7A11 is not different between HC and SLE neutrophils.
a. Heatmap visualization of RNA-seq analysis on differentially expressed ferroptosis-related genes (Standardized with GAPDH) in neutrophils between new onset treatment-naïve SLE patients (n = 6) and HCs (n = 6). b. Western blot validation for SLC7A11 antibody. 293 T cells were transfected with Slc7a11 overexpression plasmid and cells without transfection were used as control. c. Western blot assay shows the expression of cystine transporter SLC7A11 in neutrophils from HCs (n = 8) and SLE patients (n = 8). Data are shown as mean ± SD. ns p > 0.05. Two-tailed unpaired Student’s t-test was applied.
Extended Data Fig. 6 GPX4 reduction was observed in neutrophils but not other immune cells in SLE.
a. Flow cytometry quantification of GPX4 expressions in HCs (n = 16) and SLE (n = 12) neutrophils. b. GPX4 expressions in neutrophils from treatment-naïve SLE patients correlated negatively with disease activities as measured by SLEDAI (n = 12). c. Flow cytometry quantification of GPX4 expressions in lymphocytes (including CD4 + T, CD8 + T, and B cells) and monocytes from HCs (n = 11) and SLE patients (n = 9). d-e. Western blot analysis of GPX4 expressions in lymphocytes and monocytes from HCs (n = 11) and SLE patients (n = 10). f-g. Western blot analysis of GPX4 expression in HC neutrophils, monocytes, and lymphocytes (n = 7). h-i. Western blot analysis of GPX4 expression in HC neutrophils, monocytes, and lymphocytes (n = 7) cultured with 20% HC or SLE serum for 30 hours. j-k. Western blot analysis of GPX4 expression in HC neutrophils (n = 3) when cultured with 20% HC serum or SLE serum supplemented with Cl-amidine (Cl, 100 μM), APX-115 (APX) (pan-NADPH oxidase (NOX) inhibitor, 20 μM), and GSK2795039 (GSK) (NOX2 inhibitor, 10 μM). Data are shown as mean ± SD. ns p > 0.05. Two-tailed unpaired Student’s t-test was applied.
Extended Data Fig. 7 FcγR3β is essential for the SLE IgG-mediated GPX4 downregulation in neutrophils.
a-b. Expression correlation analysis between different TLRs or FcRs with GPX4 based on RNA-seq data. In SLE neutrophils, (a) TLR signaling pathways are not associated with GPX4 reduction. (b) Fcγr3b but not other FcRs’ expression is negatively associated with GPX4 reduction. c. Different Fc receptor expressions in HCs (n = 6) and SLE (n = 6) analyzed by RNA-seq. d. Western blot analysis of FcγR3β expressions in neutrophils, monocytes and lymphocytes from HCs (n = 3). e. GPX4 expressions in HL60 cells after overexpression of FcγR3β (n=4). Control referred to cells without transfection. Data are presented as mean ± SD or median with interquartile range. ns p > 0.05. one-tailed or two-tailed unpaired Student’s t-test or Mann Whitney test was applied.
Extended Data Fig. 8 Mice with Gpx4 haploinsufficiency in neutrophils developed spontaneous lupus-like disease, while Gpx4 fl/flLysMCre+ mice exhibited mild autoimmunity.
a-b. Flow cytometry quantification and western blot analysis of GPX4 in neutrophils (CD45+CD11b+Ly6G&Ly6C+) and non-neutrophils (including monocytes and lymphocytes) from Gpx4fl/fl (n = 6) and Gpx4fl/wtLysMCre+ (n = 9) mice. c-d. Flow cytometry analysis of peripheral neutrophils (CD45+CD11b+Ly6G&Ly6C+) and monocytes (CD45+CD11b+Ly6G&Ly6C-) from Gpx4fl/fl (c: n = 6, d: n = 10) and Gpx4fl/wtLysMCre+ (c: n = 9, d: n = 13) mice. e. Flow cytometry quantification of lipid-ROS and cell viability in neutrophils (n = 6) from Gpx4fl/wtLysMCre+ mice cultured in complete RPMI 1640 basic medium in the presence or absence of LPX-1 (1 μM). f. Skin lesions of Gpx4fl/wtLysMCre+ mice. g. Immunofluorescent images of glomeruli in Gpx4fl/fl mice and Gpx4fl/wtLysMCre+ mice. IgG (red), IgM (yellow), C1q (green), and DAPI (blue). h. Dot plots show the proteinuria of Gpx4fl/fl and Gpx4 fl/flLysMCre+ mice at 4 months of age assessed by BCA assay. i. ELISA assay shows the levels of serum complement 3 in Gpx4fl/fl (n = 8) and Gpx4 fl/flLysMCre+ (n = 8) mice at 6 months of age. j. ELISA assay shows the levels of serum anti-dsDNA antibodies in Gpx4fl/fl (n = 8) and Gpx4 fl/flLysMCre+ (n = 8) mice at 6 months of age. The scale bar represents 50 μm. Data are shown as mean ± SD, ns p > 0.05. Two-tailed unpaired or paired Student’s t-test was applied.
Extended Data Fig. 9 IFNα and SLE IgG enhanced ferroptosis by promoting binding of CREM to the Gpx4 promoter.
a. Western blot analysis of CREMα and CaMKIV in cytoplasm and nucleus of neutrophils from HCs and SLE patients. b. Dot plots show the CHIP analysis results on CREMα binding to the promoter of Gpx4 from neutrophils (n = 6) with indicated treatment: IFN-α (10^5 U ml-1), anti-IFNAR (10 µg ml-1), SLE IgG (2.4 g L-1) or SLE sera with IgG depletion. c-d. Efficiency of CREMα knockdown by siRNA (n = 3) or CREMα over-expression (n = 4) in HL-60 cells validated by qPCR (c) and western blot (d). e. Effect of IFN-α or SLE IgG on GPX4 expressions in HL60 cells after knockdown or overexpression of CREMα. f. Efficiency of CREMα knockdown or overexpression on ferroptosis in HL60 cells (n = 4), assessed by flow cytometry using BODIPY C11. Data are shown as mean ± SD, ns p > 0.05. Two-tailed unpaired or paired Student’s t-test was applied.
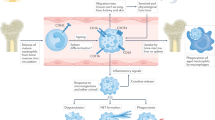
Extended Data Fig. 10 The hypothetical model for neutrophil ferroptosis in SLE pathogenesis.
Autoantibodies and interferon-α present in SLE sera enhance binding of the transcriptional repressor CREMα to Gpx4 promoter, which leads to suppressed expression of GPX4 and subsequent elevation of lipid-ROS. These lead to neutrophil ferroptosis and further promote SLE progression in patients. Moreover, mice with neutrophil-specific Gpx4 haploinsufficiency develop lupus phenotype and inhibition of neutrophil ferroptosis significantly mitigates disease development in lupus-prone mice.
Supplementary information
Supplementary Information
Supplementary Tables 1–7 and associated legends.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 5
Unprocessed western blots and/or gels.
Source Data Fig. 6
Statistical source data.
Source Data Fig. 7
Statistical source data.
Source Data Fig. 7
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 1
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 4
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 5
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 6
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 7
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 8
Statistical source data.
Source Data Extended Data Fig. 8
Unprocessed western blots and/or gels.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 9
Unprocessed western blots and/or gels.
Rights and permissions
About this article
Cite this article
Li, P., Jiang, M., Li, K. et al. Glutathione peroxidase 4–regulated neutrophil ferroptosis induces systemic autoimmunity. Nat Immunol 22, 1107–1117 (2021). https://doi.org/10.1038/s41590-021-00993-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-021-00993-3
This article is cited by
-
A follow-up study on factors affecting the recovery of patients with hypothyroidism in different selenium environments
BMC Endocrine Disorders (2024)
-
Role of ferroptosis in chronic kidney disease
Cell Communication and Signaling (2024)
-
S-nitrosothiol homeostasis maintained by ADH5 facilitates STING-dependent host defense against pathogens
Nature Communications (2024)
-
Analysis and identification of oxidative stress-ferroptosis related biomarkers in ischemic stroke
Scientific Reports (2024)
-
GSTM3 enhances radiosensitivity of nasopharyngeal carcinoma by promoting radiation-induced ferroptosis through USP14/FASN axis and GPX4
British Journal of Cancer (2024)