Abstract
Despite evidence of chronic inflammation in myelodysplastic syndrome (MDS) and cell-intrinsic dysregulation of Toll-like receptor (TLR) signaling in MDS hematopoietic stem and progenitor cells (HSPCs), the mechanisms responsible for the competitive advantage of MDS HSPCs in an inflammatory milieu over normal HSPCs remain poorly defined. Here, we found that chronic inflammation was a determinant for the competitive advantage of MDS HSPCs and for disease progression. The cell-intrinsic response of MDS HSPCs, which involves signaling through the noncanonical NF-κB pathway, protected these cells from chronic inflammation as compared to normal HSPCs. In response to inflammation, MDS HSPCs switched from canonical to noncanonical NF-κB signaling, a process that was dependent on TLR-TRAF6-mediated activation of A20. The competitive advantage of TLR-TRAF6-primed HSPCs could be restored by deletion of A20 or inhibition of the noncanonical NF-κB pathway. These findings uncover the mechanistic basis for the clonal dominance of MDS HSPCs and indicate that interfering with noncanonical NF-κB signaling could prevent MDS progression.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
References
Nimer, S. D. Myelodysplastic syndromes. Blood 111, 4841–4851 (2008).
Nilsson, L. et al. Involvement and functional impairment of the CD34+CD38−Thy-1+ hematopoietic stem cell pool in myelodysplastic syndromes with trisomy 8. Blood 100, 259–267 (2002).
Nilsson, L. et al. Isolation and characterization of hematopoietic progenitor/stem cells in 5q-deleted myelodysplastic syndromes: evidence for involvement at the hematopoietic stem cell level. Blood. 96, 2012–2021 (2000).
Muto, T. et al. Concurrent loss of Ezh2 and Tet2 cooperates in the pathogenesis of myelodysplastic disorders. J. Exp. Med. 210, 2627–2639 (2013).
Fang, J. et al. Ubiquitination of hnRNPA1 by TRAF6 links chronic innate immune signaling with myelodysplasia. Nat. Immunol. 18, 236–245 (2017).
Thanopoulou, E. et al. Engraftment of NOD/SCID-β2 microglobulin null mice with multilineage neoplastic cells from patients with myelodysplastic syndrome. Blood 103, 4285–4293 (2004).
Oishi, Y. & Manabe, I. Macrophages in age-related chronic inflammatory diseases. NPJ Aging Mech. Disease 2, 16018 (2016).
Pietras, E. M. Inflammation: a key regulator of hematopoietic stem cell fate in health and disease. Blood 130, 1693–1698 (2017).
Barreyro, L., Chlon, T. M. & Starczynowski, D. T. Chronic immune response dysregulation in MDS pathogenesis. Blood 132, 1553–1560 (2018).
Varney, M. E. et al. Loss of Tifab, a del(5q) MDS gene, alters hematopoiesis through derepression of Toll-like receptor–TRAF6 signaling. J. Exp. Med. 212, 1967–1985 (2015).
Starczynowski, D. T. et al. Identification of miR-145 and miR-146a as mediators of the 5q- syndrome phenotype. Nat. Med. 16, 49–58 (2010).
Starczynowski, D. T. et al. Genome-wide identification of human microRNAs located in leukemia-associated genomic alterations. Blood 117, 595–607 (2011).
Rhyasen, G. W. et al. Targeting IRAK1 as a therapeutic approach for myelodysplastic syndrome. Cancer Cell 24, 90–104 (2013).
Fang, J. et al. TRAF6 mediates basal activation of NF-κB necessary for hematopoietic stem cell homeostasis. Cell Rep. 22, 1250–1262 (2018).
Varney, M. E. et al. Epistasis between TIFAB and miR-146a: neighboring genes in del(5q) myelodysplastic syndrome. Leukemia 31, 491–495 (2017).
Sato, S. et al. Toll/IL-1 receptor domain-containing adaptor inducing IFN-β (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-κB and IFN-regulatory factor-3, in the Toll-like receptor signaling. J. Immunol. 171, 4304–4310 (2003).
Gohda, J., Matsumura, T. & Inoue, J. Cutting edge: TNFR-associated factor (TRAF) 6 is essential for MyD88-dependent pathway but not Toll/IL-1 receptor domain-containing adaptor-inducing IFN-β (TRIF)-dependent pathway in TLR signaling. J Immunol. 173, 2913–2917 (2004).
Schuettpelz, L. G. & Link, D. C. Regulation of hematopoietic stem cell activity by inflammation. Front. Immunol. 4, 204 (2013).
Zhao, J. L. et al. NF-κB dysregulation in microRNA-146a-deficient mice drives the development of myeloid malignancies. Proc. Natl Acad. Sci. USA 108, 9184–9189 (2011).
Takizawa, H. et al. Pathogen-Induced TLR4-TRIF Innate Immune Signaling in Hematopoietic Stem Cells Promotes Proliferation but Reduces Competitive Fitness. Cell Stem Cell 21, 225–240.e5 (2017).
Zhang, H. et al. Sepsis induces hematopoietic stem cell exhaustion and myelosuppression through distinct contributions of TRIF and MYD88. Stem Cell Rep. 6, 940–956 (2016).
Esplin, B. L. et al. Chronic exposure to a TLR ligand injures hematopoietic stem cells. J. Immunol. 186, 5367–5375 (2011).
Liu, A. et al. Cutting edge: hematopoietic stem cell expansion and common lymphoid progenitor depletion require hematopoietic-derived, cell-autonomous TLR4 in a model of chronic endotoxin. J. Immunol. 195, 2524–2528 (2015).
Chavakis, T., Mitroulis, I. & Hajishengallis, G. Hematopoietic progenitor cells as integrative hubs for adaptation to and fine-tuning of inflammation. Nat. Immunol. 20, 802–811 (2019).
Waterstrat, A., Liang, Y., Swiderski, C. F., Shelton, B. J. & Van Zant, G. Congenic interval of CD45/Ly-5 congenic mice contains multiple genes that may influence hematopoietic stem cell engraftment. Blood 115, 408–417 (2010).
Fang, J. et al. Myeloid malignancies with chromosome 5q deletions acquire a dependency on an intrachromosomal NF-κB gene network. Cell Rep. 8, 1328–1338 (2014).
Sanz, C., Richard, C., Prosper, F. & Fernandez-Luna, J. L. Nuclear factor κ B is activated in myelodysplastic bone marrow cells. Haematologica 87, 1005–1006 (2002).
Wei, Y. et al. Global H3K4me3 genome mapping reveals alterations of innate immunity signaling and overexpression of JMJD3 in human myelodysplastic syndrome CD34+ cells. Leukemia 27, 2177–2186 (2013).
Magness, S. T. et al. In vivo pattern of lipopolysaccharide and anti-CD3-induced NF-κB activation using a novel gene-targeted enhanced GFP reporter gene mouse. J. Immunol. 173, 1561–1570 (2004).
Sun, S. C. The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 17, 545–558 (2017).
Yamaguchi, N., Oyama, M., Kozuka-Hata, H. & Inoue, J. Involvement of A20 in the molecular switch that activates the non-canonical NF-κB pathway. Sci. Rep. 3, 2568 (2013).
Wertz, I. E. et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-κB signalling. Nature 430, 694–699 (2004).
Lee, E. G. et al. Failure to regulate TNF-induced NF-κB and cell death responses in A20-deficient mice. Science 289, 2350–2354 (2000).
Bird, L. TET2: the terminator. Nat. Rev. Immunol. 15, 598 (2015).
Zhang, Q. et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature. 525, 389–393 (2015).
Cai, Z. et al. Inhibition of inflammatory signaling in Tet2 mutant preleukemic cells mitigates stress-induced abnormalities and clonal hematopoiesis. Cell Stem Cell 23, 833–849 (2018).
Rhyasen, G. W. et al. An MDS xenograft model utilizing a patient-derived cell line. Leukemia 28, 1142–1145 (2014).
Kumar, M. S. et al. Coordinate loss of a microRNA and protein-coding gene cooperate in the pathogenesis of 5q-syndrome. Blood 118, 4666–4673 (2011).
Nakagawa, M. M., Thummar, K., Mandelbaum, J., Pasqualucci, L. & Rathinam, C. V. Lack of the ubiquitin-editing enzyme A20 results in loss of hematopoietic stem cell quiescence. J. Exp. Med. 212, 203–216 (2015).
Cull, A. H., Snetsinger, B., Buckstein, R., Wells, R. A. & Rauh, M. J. Tet2 restrains inflammatory gene expression in macrophages. Exp. Hematol. 55, 56–70.e13 (2017).
Ma, S. et al. Epigenetic regulator CXXC5 recruits DNA demethylase Tet2 to regulate TLR7/9-elicited IFN response in pDCs. J. Exp. Med. 214, 1471–1491 (2017).
Leoni, C. et al. Dnmt3a restrains mast cell inflammatory responses. Proc. Natl Acad. Sci. USA 114, E1490–E1499 (2017).
Li, X. et al. Methyltransferase Dnmt3a upregulates HDAC9 to deacetylate the kinase TBK1 for activation of antiviral innate immunity. Nat. Immunol. 17, 806–815 (2016).
Lee, S. C. et al. Synthetic lethal and convergent biological effects of cancer-associated spliceosomal gene mutations. Cancer Cell 34, 225–241.e8 (2018).
Smith, M. A. et al. U2AF1 mutations induce oncogenic IRAK4 isoforms and activate innate immune pathways in myeloid malignancies. Nat. Cell Biol. 21, 640–650 (2019).
Pollyea, D. A. et al. Myelodysplastic syndrome-associated spliceosome gene mutations enhance innate immune signaling. Haematologica 104, e388–e392 (2019).
Ulas, T. et al. S100-alarmin-induced innate immune programming protects newborn infants from sepsis. Nat. Immunol. 18, 622–632 (2017).
Shi, H. et al. Chemokine (C-X-C motif) ligand 1 and CXCL2 produced by tumor promote the generation of monocytic myeloid-derived suppressor cells. Cancer Sci. 109, 3826–3839 (2018).
Tavares, R. M. et al. The ubiquitin modifying enzyme A20 restricts B cell survival and prevents autoimmunity. Immunity 33, 181–191 (2010).
Fang, J. et al. A calcium- and calpain-dependent pathway determines the response to lenalidomide in myelodysplastic syndromes. Nat. Med. 22, 727–734 (2016).
Haeussler, M. et al. Evaluation of off-target and on-target scoring algorithms and integration into the guide RNA selection tool CRISPOR. Genome Biol. 17, 148 (2016).
Choi, K. & Ratner, N. iGEAK: an interactive gene expression analysis kit for seamless workflow using the R/shiny platform. BMC Genomics 20, 177 (2019).
Pellagatti, A. et al. Deregulated gene expression pathways in myelodysplastic syndrome hematopoietic stem cells. Leukemia 24, 756–764 (2010).
Gerstung, M. et al. Combining gene mutation with gene expression data improves outcome prediction in myelodysplastic syndromes. Nat. Commun. 6, 5901 (2015).
Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005).
Acknowledgements
This work was supported in parts by the National Institutes of Health (grant nos. R35HL135787, R01DK102759, R01DK113639 to D.T.S.), Cancer Free Kids (D.T.S.), Cincinnati Children’s Hospital Research Foundation (D.T.S.), The Uehara Memorial Foundation (T.M.), The Waksman Foundation of Japan (T.M.), The Mochida Memorial Foundation for Medical and Pharmaceutical Research (T.M.), Japan Society for the Promotion of Science (T.M.) and Ohio State University Comprehensive Cancer Center (T.M.). T.M. is a Leukemia and Lymphoma Society Special Fellow. D.T.S. is a Leukemia and Lymphoma Society Scholar. We thank J. Bailey and V. Summey for assistance with transplantations (Comprehensive Mouse and Cancer Core at CCHMC), and M.-D. Filippi, D. Lucas, D. Reynaud and the Starczynowski laboratory for insightful suggestions and feedback.
Author information
Authors and Affiliations
Contributions
T.M. and D.T.S. contributed to study conception and design. T.M., C.S.W., K.C., K.H. and M.S. acquired, analyzed and interpreted data. Z.G. and G.G.-M. provided samples. A.M. and Y.Z. provided reagents. T.M. and D.T.S. wrote and revised the manuscript.
Corresponding author
Ethics declarations
Competing interests
D.T.S. serves on the scientific advisory board at Kurome Therapeutics. All other authors declare no competing interests.
Additional information
Peer review information Ioana Visan was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
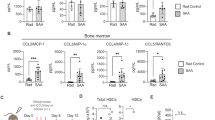
Extended Data Fig. 1 Inflammatory and immune pathway activation in MDS cells.
a, Survival analysis of MDS patients based on TRAF6 expression (probe: 227264_at) in CD34+ cells (GSE58831). Patients were stratified based on TRAF6 mRNA expression (top 20%, n = 28; bottom 20%, n = 26). Log-rank (Mantel-Cox) test. b, Overview of experimental design to examine inflammatory states in MDS and human TLR-TRAF6 primed HSPC. c, Human miR-146a deficient (miR146a-/-) and control (WT) CD34+ BM cells generated from healthy CD34+ BM using CRISPR-Cas9 gene editing or Vav-TRAF6 and WT LSK BM cells were stimulated in vitro for 90 min with 1 µg/mL of LPS (or PBS) (n = 3 each per group) and then examined for differential gene expression by RNA-sequencing. The inflammatory state for each group was determined using the GSEA. NES, normalized enrichment score. d, Expression of miR-146a in miR-146a deficient (miR146a-/-) and control (sg-CTL) CD34+ BM cells gene edited using CRISPR-Cas9. Results are presented as mean ± s.e.m. for n = 3 technical replicate samples. e, Immunoblot analysis of TRAF6 and IRAK1, two miR-146a targets, in miR146a-/- and control (sg-CTL) CD34+ BM cells gene edited using CRISPR-Cas9. Shown is an immunoblot from a single biological replicate.
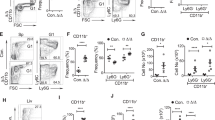
Extended Data Fig. 2 Evaluation of Vav-TRAF6-YFP and WT-YFP versus WT BM cell competition with LD-LPS.
a, Overview of experimental design to directly measure hematopoietic cell competition in the presence of low-dose chronic inflammation. Vav-TRAF6 CD45.2 BM cells (co-expressing a YFP transgene referred to as Vav-TRAF6-YFP) and WT CD45.2 BM cells were transplanted in equal proportions into lethally irradiated recipient mice. Two months after transplantation, chimeric mice were treated with LD-LPS (1 µg/g) or vehicle twice a week for 30 days and then examined for hematopoietic contribution of Vav-TRAF6-YFP and WT cells in the PB and BM. After the last LPS treatment, BM cells were serially transplanted into lethally irradiated recipient mice. b, Overview of experimental design to directly measure hematopoietic cell competition in the presence of low-dose chronic inflammation. Wild-type (WT) CD45.2 BM cells (co-expressing a YFP transgene) and WT CD45.2 BM cells were transplanted in equal proportions into lethally irradiated recipient mice. Two months after transplantation, chimeric mice were treated with LD-LPS (1 µg/g) or vehicle twice a week for 4 weeks and then examined for hematopoietic contribution of WT-YFP and WT cells in the BM. c, Representative flow cytometric profiles and gating strategy of YFP+ (WT-YFP) cells in the BM of chimeric mice after LPS or vehicle (PBS) treatment. d, The proportion of YFP+ (WT-YFP) cells in LSK populations 4 weeks after treatment with LPS or vehicle (PBS). Data represent the mean ± s.e.m., n = 6 mice per group.
Extended Data Fig. 3 Overexpression of TRAF6 alters the response of hematopoietic progenitor cells to LD-LPS.
a, Overview of experimental design to examine the long-term effects of low-dose chronic inflammation on hematopoiesis. Vav-TRAF6 (T6) CD45.2 BM cells or WT CD45.2 BM cells were isolated from mice treated with LD-LPS (1 µg/g) or vehicle twice a week for 30 days and then transplanted with a ratio of 10:1 of CD45.1 competitor BM cells into lethally irradiated recipient mice. Three months after transplantation, BM cells were serially transplanted into lethally irradiated recipient mice. b, The proportion of donor-derived CD45.2+ cells in MPP2 (Flk2-CD150+CD48+LSK), MPP3 (Flk2-CD150-CD48+LSK), and MPP4 (Flk2+CD150-CD48+LSK) after tertiary transplantation. Results are presented as mean ± s.e.m. for n = 3 mice per group. Statistical analysis was performed by a two-tailed Student’s t-test. *, P < 0.05.
Extended Data Fig. 4 TLR-TRAF6 primed HSPC exhibit expression of non-canonical NF-κB gene signatures.
a, Normalized enrichment scores (NES) and P value of gene signatures established from WT and Vav-TRAF6 LSK stimulated with LPS evaluated in constitutively active (ca) NIK expressing LSK (GSE88949). b, GSEA plots established from caNIK LSK were evaluated in miR-146a deficient (miR146a-/-) CD34+ BM cells gene edited using CRISPR-Cas9 and then stimulated with 1 µg/mL of LPS (or PBS) for 90 min. The gene expression profiles are relative to unstimulated miR-146a-/- CD34+ BM and control (sg-CTL) (+/− LPS). (c) Capillary immunoassay of CD34+ cells isolated from healthy controls or MDS BM visualized by chemiluminescence using ProteinSimple. Shown is an immunoassay from a single biological replicate.
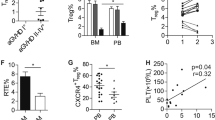
Extended Data Fig. 5 TET2 deficiency in hematopoietic cells results in increased myeloid-biased hematopoiesis without affecting the proportions of HSC after LD-LPS.
a, Overview of experimental design to examine the effects of low-dose chronic inflammation on hematopoiesis. Tet2f/f VavCre CD45.2 BM cells (Tet2-/-) or Tet2f/f (WT) CD45.2 BM cells were isolated from mice treated with LD-LPS (1 µg/g) or vehicle twice a week for 4 weeks and then transplanted into lethally irradiated recipient mice (along with CD45.1 competitor BM cells). One month after transplantation, PB and BM cells were evaluated by flow cytometry. b, The proportion of donor-derived CD45.2+ myeloid (CD11b+) and lymphoid (B220+ and CD3+) cells in the PB (n = 6 mice per group). * P = 0.02. c, The proportion of donor-derived CD45.2+ LSK and LT-HSC in the BM of mice after treatment with LD-LPS. Results are presented as mean ± s.e.m., n = 6 for all groups; n = 3 for WT LPS treated group. * P = 0.03. Statistical analysis in b was performed by a two-tailed Student’s t-test. Statistical analysis in c was performed by a one-tailed Student’s t-test.
Extended Data Fig. 6 Generation of Traf6- and Tet2-deficient mice.
Genotyping analysis of Tet2-/- VavCre and Traf6-/- Tet2-/- VavCre mice.
Extended Data Fig. 7 Generation of Vav-TRAF6 and A20-deficient mice.
Genotyping analysis of Vav-TRAF6 RosaCreER and A20-/- Vav-TRAF6 RosaCreER mice. A20 floxed allele recombination is shown after Tamoxifen treatment.
Extended Data Fig. 8 A20 knockdown impairs MDSL and THP1 cell function.
a, Immunoblotting of MDSL cells expressing independent shRNAs targeting A20 (shA20) or non-targeting shRNA (shControl). Shown is an immunoblot from a single biological replicate. b, Colony forming potential of MDSL cells expressing shRNAs targeting A20 (shA20) or non-targeting shRNA (shControl) in methylcellulose. Results are presented as mean ± s.e.m., for n = 3 independent biological replicates. * P = 0.006, ** P = 0.0004. c, Immunoblotting of THP1 cells expressing an shRNA targeting A20 (shA20) or non-targeting shRNA (shControl). Shown is an immunoblot from a single biological replicate. d, Colony forming potential of THP1 cells expressing shRNAs targeting A20 or non-targeting shRNA (shControl) in methylcellulose. Results are presented as mean ± s.e.m., for n = 3 independent biological replicates. *, P = 0.0001. Statistical analysis in b,d was performed by a two-tailed Student’s t-test.
Supplementary information
Supplementary Tables
Supplementary Tables 1–11.
Source data
Source Data Fig. 2
Statistical Source Data for Fig. 2
Source Data Fig. 3
Statistical Source Data for Fig. 3
Source Data Fig. 4
Statistical Source Data for Fig. 4
Source Data Fig. 5
Statistical Source Data for Fig. 5
Source Data Fig. 6
Statistical Source Data for Fig. 6
Source Data Fig. 7
Statistical Source Data for Fig. 7
Source Data Extended Data Fig. 3
Statistical Source Data for ED Fig. 3
Source Data Extended Data Fig. 5
Statistical Source Data for ED Fig. 5
Source Data Extended Data Fig. 8
Statistical Source Data for ED Fig. 8
Source Data Fig. 4
Unprocessed western blots
Source Data Fig. 5
Unprocessed western blots
Source Data Fig. 6
Unprocessed western blots
Source Data Fig. 7
Unprocessed western blots
Source Data Extended Data Fig. 1
Unprocessed western blots
Source Data Extended Data Fig. 8
Unprocessed western blots
Rights and permissions
About this article
Cite this article
Muto, T., Walker, C.S., Choi, K. et al. Adaptive response to inflammation contributes to sustained myelopoiesis and confers a competitive advantage in myelodysplastic syndrome HSCs. Nat Immunol 21, 535–545 (2020). https://doi.org/10.1038/s41590-020-0663-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-020-0663-z
This article is cited by
-
Metabolic reprogramming regulated by TRAF6 contributes to the leukemia progression
Leukemia (2024)
-
Targeting PD-1/PD-L1 pathway in myelodysplastic syndromes and acute myeloid leukemia
Experimental Hematology & Oncology (2022)
-
Myeloid neoplasms and clonal hematopoiesis from the RUNX1 perspective
Leukemia (2022)
-
Cooperation between KDM6B overexpression and TET2 deficiency in the pathogenesis of chronic myelomonocytic leukemia
Leukemia (2022)
-
Mechanisms involved in hematopoietic stem cell aging
Cellular and Molecular Life Sciences (2022)