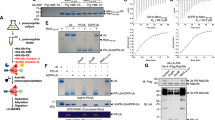
Abstract
Ubiquitination plays essential roles in eukaryotic cellular processes. The effector protein CteC from Chromobacterium violaceum blocks host ubiquitination by mono-ADP-ribosylation of ubiquitin (Ub) at residue T66. However, the structural basis for this modification is unknown. Here we report three crystal structures of CteC in complexes with Ub, NAD+ or ADP-ribosylated Ub, which represent different catalytic states of CteC in the modification. CteC adopts a special ‘D-E’ catalytic motif for catalysis and binds NAD+ in a half-ligand binding mode. The specific recognition of Ub by CteC is determined by a relatively separate Ub-targeting domain and a long loop L6, not the classic ADP-ribosylating turn–turn loop. Structural analyses with biochemical results reveal that CteC represents a large family of poly (ADP-ribose) polymerase (PARP)-like ADP-ribosyltransferases, which harbors chimeric features from the R-S-E and H-Y-E classes of ADP-ribosyltransferases. The family of CteC-like ADP-ribosyltransferases has a common ‘D-E’ catalytic consensus and exists extensively in bacteria and eukaryotic microorganisms.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The coordinates and structural factors of the SeMet-labeled CteC–Ub, native CteC–Ub, CteC–Ub–NAD+ and CteC E220A–Ub–NAD+ complexes have been deposited in the Protein Data Bank under accession numbers 8HTC, 8HTD, 8HTF and 8HTE, respectively. Source data are provided with this paper.
References
Komander, D. & Rape, M. The ubiquitin code. Annu. Rev. Biochem. 81, 203–229 (2012).
Jiang, X. & Chen, Z. J. The role of ubiquitylation in immune defence and pathogen evasion. Nat. Rev. Immunol. 12, 35–48 (2011).
Galan, J. E. Common themes in the design and function of bacterial effectors. Cell Host Microbe 5, 571–579 (2009).
Zhou, Y. & Zhu, Y. Diversity of bacterial manipulation of the host ubiquitin pathways. Cell Microbiol. 17, 26–34 (2015).
Mukherjee, R. & Dikic, I. Regulation of host–pathogen interactions via the ubiquitin system. Annu. Rev. Microbiol 76, 211–233 (2022).
Rohde, J. R., Breitkreutz, A., Chenal, A., Sansonetti, P. J. & Parsot, C. Type III secretion effectors of the IpaH family are E3 ubiquitin ligases. Cell Host Microbe 1, 77–83 (2007).
Zhu, Y. et al. Structure of a Shigella effector reveals a new class of ubiquitin ligases. Nat. Struct. Mol. Biol. 15, 1302–1308 (2008).
Wan, M. et al. A bacterial effector deubiquitinase specifically hydrolyses linear ubiquitin chains to inhibit host inflammatory signalling. Nat. Microbiol. 4, 1282–1293 (2019).
Mikolcevic, P., Hlousek-Kasun, A., Ahel, I. & Mikoc, A. ADP-ribosylation systems in bacteria and viruses. Comput. Struct. Biotechnol. J. 19, 2366–2383 (2021).
Luscher, B. et al. ADP-ribosylation, a multifaceted posttranslational modification involved in the control of cell physiology in health and disease. Chem. Rev. 118, 1092–1136 (2018).
Cohen, M. S. & Chang, P. Insights into the biogenesis, function, and regulation of ADP-ribosylation. Nat. Chem. Biol. 14, 236–243 (2018).
Yoshida, T. & Tsuge, H. Common mechanism for target specificity of protein- and DNA-targeting ADP-ribosyltransferases. Toxins 13, 40 (2021).
Simon, N. C., Aktories, K. & Barbieri, J. T. Novel bacterial ADP-ribosylating toxins: structure and function. Nat. Rev. Microbiol. 12, 599–611 (2014).
Yan, F. et al. Threonine ADP-ribosylation of ubiquitin by a bacterial effector family blocks host ubiquitination. Mol. Cell 78, 641–652.e9 (2020).
Bence, N. F., Sampat, R. M. & Kopito, R. R. Impairment of the ubiquitin-proteasome system by protein aggregation. Science 292, 1552–1555 (2001).
Sun, J., Maresso, A. W., Kim, J. J. & Barbieri, J. T. How bacterial ADP-ribosylating toxins recognize substrates. Nat. Struct. Mol. Biol. 11, 868–876 (2004).
Han, S. & Tainer, J. A. The ARTT motif and a unified structural understanding of substrate recognition in ADP-ribosylating bacterial toxins and eukaryotic ADP-ribosyltransferases. Int. J. Med. Microbiol. 291, 523–529 (2002).
Han, S., Arvai, A. S., Clancy, S. B. & Tainer, J. A. Crystal structure and novel recognition motif of Rho ADP-ribosylating C3 exoenzyme from Clostridium botulinum: structural insights for recognition specificity and catalysis. J. Mol. Biol. 305, 95–107 (2001).
Tsurumura, T. et al. Arginine ADP-ribosylation mechanism based on structural snapshots of iota-toxin and actin complex. Proc. Natl Acad. Sci. USA 110, 4267–4272 (2013).
Akturk, A. et al. Mechanism of phosphoribosyl-ubiquitination mediated by a single Legionella effector. Nature 557, 729–733 (2018).
Bhogaraju, S. et al. Phosphoribosylation of ubiquitin promotes serine ubiquitination and impairs conventional ubiquitination. Cell 167, 1636–1649.e13 (2016).
Kalayil, S. et al. Insights into catalysis and function of phosphoribosyl-linked serine ubiquitination. Nature 557, 734–738 (2018).
Dong, Y. et al. Structural basis of ubiquitin modification by the Legionella effector SdeA. Nature 557, 674–678 (2018).
Ting, S. Y. et al. Bifunctional immunity proteins protect bacteria against FtsZ-targeting ADP-ribosylating toxins. Cell 175, 1380–1392.e14 (2018).
Bennett, M. J., Choe, S. & Eisenberg, D. Refined structure of dimeric diphtheria toxin at 2.0 Å resolution. Protein Sci. 3, 1444–1463 (1994).
Jorgensen, R., Wang, Y., Visschedyk, D. & Merrill, A. R. The nature and character of the transition state for the ADP-ribosyltransferase reaction. EMBO Rep. 9, 802–809 (2008).
Bullen, N. P. et al. An ADP-ribosyltransferase toxin kills bacterial cells by modifying structured non-coding RNAs. Mol. Cell 82, 3484–3498.e11 (2022).
Jurėnas, D. et al. Photorhabdus antibacterial Rhs polymorphic toxin inhibits translation through ADP-ribosylation of 23S ribosomal RNA. Nucleic Acids Res. 49, 8384–8395 (2021).
Jurėnas, D. et al. Mounting, structure and autocleavage of a type VI secretion-associated Rhs polymorphic toxin. Nat. Commun. 12, 6998 (2021).
Suskiewicz, M. J. et al. HPF1 completes the PARP active site for DNA damage-induced ADP-ribosylation. Nature 579, 598–602 (2020).
Langelier, M. F., Zandarashvili, L., Aguiar, P. M., Black, B. E. & Pascal, J. M. NAD+ analog reveals PARP-1 substrate-blocking mechanism and allosteric communication from catalytic center to DNA-binding domains. Nat. Commun. 9, 844 (2018).
van Kempen, M. et al. Fast and accurate protein structure search with Foldseek. Nat. Biotechnol. https://doi.org/10.1038/s41587-023-01773-0 (2023).
Varadi, M. et al. AlphaFold protein structure database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 50, D439–D444 (2022).
Prokhorova, E. et al. Serine-linked PARP1 auto-modification controls PARP inhibitor response. Nat. Commun. 12, 4055 (2021).
Schuller, M. et al. Molecular basis for DarT ADP-ribosylation of a DNA base. Nature 596, 597–602 (2021).
LeRoux, M. et al. The DarTG toxin-antitoxin system provides phage defence by ADP-ribosylating viral DNA. Nat. Microbiol. 7, 1028–1040 (2022).
Lang, A. E. et al. Photorhabdus luminescens toxins ADP-ribosylate actin and RhoA to force actin clustering. Science 327, 1139–1142 (2010).
Pfaumann, V., Lang, A. E., Schwan, C., Schmidt, G. & Aktories, K. The actin and Rho-modifying toxins PTC3 and PTC5 of Photorhabdus luminescens: enzyme characterization and induction of MAL/SRF-dependent transcription. Cell Microbiol. 17, 579–594 (2015).
Belyy, A. et al. Mechanism of threonine ADP-ribosylation of F-actin by a Tc toxin. Nat. Commun. 13, 4202 (2022).
Minor, W., Cymborowski, M., Otwinowski, Z. & Chruszcz, M. HKL-3000: the integration of data reduction and structure solution—from diffraction images to an initial model in minutes. Acta Crystallogr. D Biol. Crystallogr. 62, 859–866 (2006).
Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Potterton, L. et al. CCP4i2: the new graphical user interface to the CCP4 program suite. Acta Crystallogr. D Struct. Biol. 74, 68–84 (2018).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Williams, C. J. et al. MolProbity: more and better reference data for improved all-atom structure validation. Protein Sci. 27, 293–315 (2018).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Mirdita, M. et al. ColabFold: making protein folding accessible to all. Nat. Methods 19, 679–682 (2022).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Nguyen, L. T., Schmidt, H. A., von Haeseler, A. & Minh, B. Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274 (2015).
Letunic, I. & Bork, P. Interactive Tree Of Life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res. 49, W293–W296 (2021).
Acknowledgements
We thank the core facility of Life Sciences Institute of Zhejiang University for equipment support and the staff at beamlines BL17U1, BL18U1 and BL19U1 of SSRF for assistance in diffraction data collection. This work was supported by grants from the Fundamental Research Funds for the Central Universities (226-2022-00229), NSFC (81925024 to Y. Zhu; 82002103 to X.W.), Zhejiang NSF (LR20H190001 to Y. Zhou), the National Science and Technology Major Project (2017YFA0503900 to Y. Zhu) and China Postdoctoral Science Foundation (2022M722779 to J.T.). Y. Zhu and Y. Zhou were supported by the National High-level Talents Special Support Program of China.
Author information
Authors and Affiliations
Contributions
Y. Zhou and Y. Zhu conceived and supervised the study. J.T., Y.X., X.W., F.Y., Y. Zhu and Y. Zhou designed experiments. J.T. and X.W. performed the structural studies. J.T., Y.X. and F.Y. carried out the biochemical and cell biological assays. W.X. and X.L. performed the mass spectrometric analyses. Y.C. prepared the reagents. J.T., Y.X., F.Y., Y. Zhu and Y. Zhou analyzed the data. J.T., Y.X., Y. Zhu and Y. Zhou wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Yuxin Mao, John Whitney and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
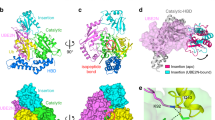
Extended Data Fig. 1 Structural topology of CteC.
a, Schematic diagram of threonine ADP-ribosylation of Ub by CteC. b, Domain organization of CteC. The CteC consists of an enzymatic domain (colored in green) and a Ub-targeting domain (colored in yellow). c, Structural topology of CteC. The Ub-targeting domain is composed of four helices and highlighted in yellow. d, The Fo-Fc omit electronic density map of the nicotinamide riboside and phosphate moieties of NAD+ in the structure of CteC-Ub-NAD+ complex. The map is countered at 3.0 σ. e, Sequence alignment of CteC and its family members CHBU from B. ubonensis and CHCS from Corallococcus sp. AB030. The sequence alignment was conducted in MAFFT. The secondary structures of CteC are illustrated on top of the sequences. The R-S-E and D-E motifs are highlighted in red. The Ub-interacting residues M159, M160, E169 and D175 were highlighted with asterisks.
Extended Data Fig. 2 Mutagenetic analyses of the PN and L11 loops of CteC.
a, Close-up view of the PN and L11 loops of CteC. The residues in the PN and L11 loops are labeled as indicated. The PN loop is highlighted in red. NAD+ is shown as sticks in purple. b, Isothermal titration calorimetry assays to determine the interactions of Ub with the CteC mutants of the residues in the PN and L11 loops. 0.1 mM CteC mutant protein were titrated with 1mM Ub in each assay. The affinities were calculated by fitting the integrated titration data with one binding site model in Origin 7.0. c, ε-NAD+ hydrolysis assays of the CteC mutants of the residues in the PN and L11 loops. The CteC variant was incubated with Ub at room temperature for 2 min. The reactions were then initiated by adding ε-NAD+. The fluorescence intensities were detected using a microplate reader with an excitation wavelength of 300 nm and an emission wavelength of 410 nm. d, Mutational effects of the residues in the PN and L11 loops on the CteC ART activity. The samples were analyzed via native PAGE and immunoblotting with an anti-Ub antibody.
Extended Data Fig. 3 Structural basis for specific recognition of Ub and polyUb by CteC.
a, Structural comparison of Ub with the Ub-like proteins, NEDD8 and SUMO. The surface electrostatic potential distribution of Ub, NEDD8 (PDB ID: 1NDD) and SUMO (PDB ID: 2BF8) is colored according to their electrostatic potential, with red surfaces indicating negative charges and blue surfaces indicating positive charges. The key residues for recognition by CteC in Ub, and the corresponding residues in NEDD8 and SUMO are labeled as indicated. b, Sequence alignment of Ub with NEDD8 and SUMO. The residue T66 of Ub is highlighted in green. The key CteC-interacting residues and T66 are indicated by asterisks. c, Structural superimposition of the CteC-Ub complex with diUbs of different linkages. Distal- or proximal-Ubs from K11- (PDB ID: 3NOB), K48- (PDB ID: 2KDF), K63- (PDB ID: 3WXG) and M1-linked (PDB ID: 6NJD) diUbs were superimposed with the CteC-Ub complex. The K11-, K48-, K63-, and M1-linked diUbs are colored in pink, orange, purple and blue, respectively.
Extended Data Fig. 4 Structural and mutagenetic analyses of the ‘D-E’ catalytic motif of CteC.
a, Close-up view of the residue D134 in the structural superimposition of the CteC-Ub complex and the CteC-Ub-NAD+ complex. D134 interacts with the 2'-hydroxyl group of N-ribose of NAD+ in the CteC-Ub-NAD+ complex structure. b, Mutational effects of the residues of the R-S-E and D-E motifs in CteC on yeast growth inhibition. Yeasts were grown overnight on agar plates of SD/-Trp selective medium supplemented with 2% glucose. The resulting yeast colonies were then streaked onto agar plates of SD/-Trp selective medium supplemented with 2% glucose or 2% galactose, and incubated for 3 days at 30°C to evaluate the effects of the CteC mutations on yeast growth inhibition.
Extended Data Fig. 5 The residual ART activity of CteC E220A in the long-time incubation with Ub.
a, Fo-Fc omit electronic density maps of the N-ribose-phosphate moiety on T66 of Ub and the released nicotinamide in the CteC E220A-Ub-NAD+ complex structure. The map is countered at 3.0 σ. b, The residual ART activity of the E220A mutant of CteC in the long-time in vitro assays. 5 μM CteC (top) or E220A protein (bottom) was incubated with Ub and NAD+ at a molar ratio of 1:10:10 for indicated time at 4 °C. The ADP-ribosylation of Ub was examined via mobility shifts on SDS-PAGE gels and Coomassie blue staining. c, ADP-ribosylation of Ub by CteC E220A in crystallization conditions. According to the same sample preparation procedure for crystallization of the CteC E220A-Ub-NAD+ complex, recombinant CteC E220A protein at the final concentration of 25 mg/ml was incubated with Ub and NAD+ at a molar ratio of 1:2:5 at 4°C overnight. The samples were then analyzed by 8% native PAGE and Coomassie blue staining. The modified ADPR-Ub was extracted for mass spectrometric analysis. Unmodified Ub was analyzed as a reference. Asterisks indicate degraded CteC E220A proteins. d, Mass spectrometric analyses of the T66-containing peptide (ESTADPRLHLVLR) in ADPR-Ub produced by CteC E220A. Extracted ion chromatograms of the peptides are shown. The peak intensities reflect the relative amounts of the unmodified and ADP-ribosylated peptides. e, Identification of the ADP-ribosylation site of the T66-containing peptide in ADPR-Ub produced by CteC E220A using ETD tandem mass spectrum.
Extended Data Fig. 6 Conformational changes of CteC in the CteC E220A-Ub-NAD+ complex.
a, Isothermal titration calorimetry analysis of the interaction between the CteC-N139L/P141E mutant and Ub. The CteC N139L/P141E protein (0.1 mM) was titrated with Ub (1 mM) in the assay. The affinity was calculated by fitting the integrated titration data with one binding site model. b, The conformational changes of the L3 and L6 loops of CteC in the CteC E220A-Ub-NAD+ complex. The CteC structure from the CteC-Ub-NAD+ complex was superimposed with the CteC E220A structure from the CteC E220A-Ub-NAD+ complex. NAD+ and released nicotinamide are shown as sticks in purple and blue, respectively. The enzymatic and Ub-targeting domains of CteC E220A are colored in wheat and blue, respectively. The two domains of CteC from the CteC-Ub-NAD+ complex are colored in green and yellow, respectively. c–e, Close-up view of the detailed conformational changes of the residues F133 and D134 (c), the L3 loop (d) and the L6 loop (e) at the L3-L6 interface in the CteC E220A-Ub-NAD+ complex. The conformational changes are indicated by black arrows.
Extended Data Fig. 7 Structural comparison of CteC with the R-S-E class of ARTs.
a, b, Sequence alignment of the R-S-E motif (a) and the PN loop (b) of CteC with those of C3 toxin, Iota toxin, the Legionella effector SdeA and the Serratia proteamaculans T6SS effector Tre1, which are ARTs of the R-S-E class. Sequence alignment was carried out in MAFFT. The secondary structures of CteC are illustrated under the sequences. The R-S-E motif and the residue H110 in the PN loop of CteC are highlighted as indicated. c, Structural comparison of the PN loops from CteC and C3 toxin (PDB ID: 1GZF). The NAD+-interacting residues in the PN loops are shown as sticks. d–f, Structural comparison of CteC with Iota toxin (PDB ID: 4H03) (d), SdeA (PDB ID: 5YIJ) (e) and Tre1 (PDB ID: 6DRH) (f). The structures of Iota, SdeA and Tre1 are colored in grey. The β-strands in their ART folds are colored in wheat. The PN loops are highlighted in red. NAD+ is shown as sticks.
Extended Data Fig. 8 Structural comparison of CteC with bacterial ARTs of the H-Y-E class and human PARPs.
a–d and g, Structural comparison of CteC with DT toxin (PDB ID: 1DDT) (a), ExoA toxin (PDB ID: 2ZIT) (b), RhsP2 (PDB ID: 7RT7) (c), Tre23 (predicted by AlphaFold) (d) and PARP1 (PDB ID: 6BHV) (g). The β-strands of the ART folds are colored in cyan. The Helixβ1-β2 helices between β1 and β2 are colored in blue. NAD+ and NAD+ analogs are shown as sticks. The donor loops of the ARTs are labeled as indicated. The long sequence between β4 and β5 in PARP1 is highlighted in yellow (g). RhsP2 harbors a H-F-E catalytic motif. e–f, Close-up view of CteC and DT toxin in structural superimposition. The β-strands of the ART folds in CteC and DT toxin are colored in cyan and green, respectively (e). The catalytic residues in the H-Y-E motif of DT toxin are highlighted in green. The enzymatic domain and Ub-targeting domain of CteC are colored in grey and yellow, respectively. The short loop linking β4 and β5 in DT toxin is labeled as indicated (e). The α2 helix (Helixβ1-β2) of CteC is highlighted in blue (f). NAD+ molecules in CteC and DT toxin are shown as sticks. h, i, Close-up view of the long sequence regions between β4 and β5 (h) and the Helixβ1-β2 helices (i) in PARP2 (PDB ID: 6TX3) and CteC. The structure of PARP2 is colored in grey. The long sequence region linking β4 and β5 in PARP2 is highlighted in orange. CteC is colored as in Fig. 5a (h). The Helixβ1-β2 (α2) of CteC is highlighted in blue (i). The NAD+ ligands in CteC and PARP2 are shown as sticks. j, Structure of mycobacterial DarT toxin (PDB ID: 7OMW). The β-strands of the ART fold are colored in cyan. The Helixβ1-β2 is colored in blue. The long sequence region linking β4 and β5 in DarT is highlighted in yellow. k, Structural superimposition of the C-terminal domains of CteC and DarT.
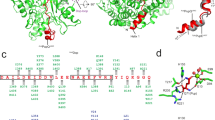
Extended Data Fig. 9 Sequence alignment of CteC with its structural homologues.
Multi-sequence alignment of the ART domains of CteC with its 21 structural homologues obtained in Foldseek was performed in MAFFT. The residues in the R-S-E and D-E motifs are labeled as indicated. The Ub-targeting domain of CteC and the variable long sequence regions linking β4 and β5 of the CteC homologues are highlighted in black boxes.
Extended Data Fig. 10 Structural analysis of the structural homologues of CteC.
The AlphaFold-predicted structures of CHAS from Acinetobacter sp. SFD (a), CHLA from Luteibacter anthropic (b), CHOS from Ochrobactrum sp. 715/2009 (c), and CHCY from Citrobacter youngae ATCC 29200 (d) are shown as cartoons and colored according to their pLDDT (predicted Local Distance Difference Test) values, ranging from blue for high confidence to red for low confidence. In addition to the ART domain, CHAS contains a C-terminal helical domain (CTD). Both CHOS and CHCY harbor a separate N-terminal domain (NTD).
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2.
Source data
Source Data Fig. 2
Unprocessed western blots and gels.
Source Data Fig. 3
Unprocessed western blots and gels.
Source Data Fig. 4
Unprocessed western blots and gels.
Source Data Fig. 6
Unprocessed western blots and gels.
Source Data Extended Data Fig. 2
Unprocessed western blots and gels.
Source Data Extended Data Fig. 5
Unprocessed gels.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tan, J., Xu, Y., Wang, X. et al. Molecular basis of threonine ADP-ribosylation of ubiquitin by bacterial ARTs. Nat Chem Biol 20, 463–472 (2024). https://doi.org/10.1038/s41589-023-01475-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-023-01475-3