Abstract
The modification of intracellular proteins with O-linked β-N-acetylglucosamine (O-GlcNAc) moieties is a highly dynamic process that spatiotemporally regulates nearly every important cellular program. Despite its significance, little is known about the substrate recognition and regulation modes of O-GlcNAc transferase (OGT), the primary enzyme responsible for O-GlcNAc addition. In this study, we identified the intervening domain (Int-D), a poorly understood protein fold found only in metazoan OGTs, as a specific regulator of OGT protein–protein interactions and substrate modification. Using proteomic peptide phage display (ProP-PD) coupled with structural, biochemical and cellular characterizations, we discovered a strongly enriched peptide motif, employed by the Int-D to facilitate specific O-GlcNAcylation. We further show that disruption of Int-D binding dysregulates important cellular programs, including response to nutrient deprivation and glucose metabolism. These findings illustrate a mode of OGT substrate recognition and offer key insights into the biological roles of this unique domain.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Atomic coordinates of the OGT4.5 co-crystal structures have been deposited in the RCSB Protein Data Bank under accession codes 8FE6 (OGT4.5:UDP-GlcNAc:CP37), 8FE7 (OGT4.5:UDP-GlcNAc:SMG9) and 8FUF (OGT4.5:UDP-GlcNAc:ZNF831). The data that support the findings of this study are available within the main text and its Supplementary Information file. Data are also available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Chiaradonna, F., Ricciardiello, F. & Palorini, R. The nutrient-sensing hexosamine biosynthetic pathway as the hub of cancer metabolic rewiring. Cells 7, 53 (2018).
Liu, C. & Li, J. O-GlcNAc: a sweetheart of the cell cycle and DNA damage response. Front. Endocrinol. 9, 415 (2018).
Parker, M. P., Peterson, K. R. & Slawson, C. O-GlcNAcylation and O-GlcNAc cycling regulate gene transcription: emerging roles in cancer. Cancers 13, 1666 (2021).
Ruan, H.-B., Nie, Y. & Yang, X. Regulation of protein degradation by O-GlcNAcylation: crosstalk with ubiquitination. Mol. Cell. Proteom. 12, 3489–3497 (2013).
Ong, Q., Han, W. & Yang, X. O-GlcNAc as an integrator of signaling pathways. Front. Endocrinol. 9, 599 (2018).
Hart, G. W. Nutrient regulation of signaling and transcription. J. Biol. Chem. 294, 2211–2231 (2019).
Kokot, T. & Köhn, M. Emerging insights into serine/threonine-specific phosphoprotein phosphatase function and selectivity. J. Cell Sci. 135, jcs259618 (2022).
Van Roey, K. et al. Short linear motifs: ubiquitous and functionally diverse protein interaction modules directing cell regulation. Chem. Rev. 114, 6733–6778 (2014).
Tompa, P., Davey, N. E., Gibson, T. J. & Babu, M. M. A million peptide motifs for the molecular biologist. Mol. Cell 55, 161–169 (2014).
Kumar, M. et al. The eukaryotic linear motif resource: 2022 release. Nucleic Acids Res. 50, D497–D508 (2022).
Lazarus, M. B., Nam, Y., Jiang, J., Sliz, P. & Walker, S. Structure of human O-GlcNAc transferase and its complex with a peptide substrate. Nature 469, 564–567 (2011).
Jínek, M. et al. The superhelical TPR-repeat domain of O-linked GlcNAc transferase exhibits structural similarities to importin α. Nat. Struct. Mol. Biol. 11, 1001–1007 (2004).
Meek, R. W. et al. Cryo-EM structure provides insights into the dimer arrangement of the O-linked β-N-acetylglucosamine transferase OGT. Nat. Commun. 12, 6508 (2021).
Iyer, S. P. N. & Hart, G. W. Roles of the tetratricopeptide repeat domain in O-GlcNAc transferase targeting and protein substrate specificity. J. Biol. Chem. 278, 24608–24616 (2003).
Levine, Z. G. et al. O-GlcNAc transferase recognizes protein substrates using an asparagine ladder in the tetratricopeptide repeat (TPR) superhelix. J. Am. Chem. Soc. 140, 3510–3513 (2018).
Joiner, C. M., Levine, Z. G., Aonbangkhen, C., Woo, C. M. & Walker, S. Aspartate residues far from the active site drive O-GlcNAc transferase substrate selection. J. Am. Chem. Soc. 141, 12974–12978 (2019).
Kositzke, A. et al. Elucidating the protein substrate recognition of O-GlcNAc transferase (OGT) toward O-GlcNAcase (OGA) using a GlcNAc electrophilic probe. Int. J. Biol. Macromol. 169, 51–59 (2021).
Joiner, C. M., Hammel, F. A., Janetzko, J. & Walker, S. Protein substrates engage the lumen of O-GlcNAc transferase’s tetratricopeptide repeat domain in different ways. Biochemistry 60, 847–853 (2021).
Davey, N. E. et al. Discovery of short linear motif-mediated interactions through phage display of intrinsically disordered regions of the human proteome. FEBS J. 284, 485–498 (2017).
Hanover, J. A., Krause, M. W. & Love, D. C. The hexosamine signaling pathway: O-GlcNAc cycling in feast or famine. Biochim. Biophys. Acta 1800, 80–95 (2010).
Benz, C. et al. Proteome-scale mapping of binding sites in the unstructured regions of the human proteome. Mol. Syst. Biol. 18, e10584 (2022).
Davey, N. E., Simonetti, L. & Ivarsson, Y. ProP-PD for proteome-wide motif-mediated interaction discovery. Trends Biochem. Sci. 47, 547–548 (2022).
Davey, N. E., Haslam, N. J., Shields, D. C. & Edwards, R. J. SLiMFinder: a web server to find novel, significantly over-represented, short protein motifs. Nucleic Acids Res. 38, W534–W539 (2010).
Edwards, R. J., Davey, N. E. & Shields, D. C. SLiMFinder: a probabilistic method for identifying over-represented, convergently evolved, short linear motifs in proteins. PLoS ONE 2, e967 (2007).
Huynh, K. & Partch, C. L. Analysis of protein stability and ligand interactions by thermal shift assay. Curr. Protoc. Protein Sci. 79, 28.9.1–28.9.14 (2015).
Yamashita, A. et al. SMG-8 and SMG-9, two novel subunits of the SMG-1 complex, regulate remodeling of the mRNA surveillance complex during nonsense-mediated mRNA decay. Genes Dev. 23, 1091–1105 (2009).
Liu, J. et al. Quantitative and site-specific chemoproteomic profiling of protein O-GlcNAcylation in the cell cycle. ACS Chem. Biol. 16, 1917–1923 (2021).
Takeda, S. et al. Role of a tyrosine phosphorylation of SMG-9 in binding of SMG-9 to IQGAP and the NMD complex. Biochem. Biophys. Res. Commun. 410, 29–33 (2011).
Jerabek-Willemsen, M. et al. MicroScale Thermophoresis: interaction analysis and beyond. J. Mol. Struct. 1077, 101–113 (2014).
Nooren, I. M. A. & Thornton, J. M. Structural characterisation and functional significance of transient protein–protein interactions. J. Mol. Biol. 325, 991–1018 (2003).
Janin, J., Bahadur, R. P. & Chakrabarti, P. Protein–protein interaction and quaternary structure. Q. Rev. Biophys. 41, 133–180 (2008).
Ardito, F., Giuliani, M., Perrone, D., Troiano, G. & Lo Muzio, L. The crucial role of protein phosphorylation in cell signaling and its use as targeted therapy (Review). Int. J. Mol. Med. 40, 271–280 (2017).
Mishra, S., Ande, S. R. & Salter, N. W. O-GlcNAc modification: why so intimately associated with phosphorylation? Cell Commun. Signal. 9, 1 (2011).
Taddei, M. L., Pardella, E., Pranzini, E., Raugei, G. & Paoli, P. Role of tyrosine phosphorylation in modulating cancer cell metabolism. Biochim. Biophys. Acta Rev. Cancer 1874, 188442 (2020).
Hunter, T. The genesis of tyrosine phosphorylation. Cold Spring Harb. Perspect. Biol. 6, a020644 (2014).
Thompson, J. W., Griffin, M. E. & Hsieh-Wilson, L. C. Methods for the detection, study, and dynamic profiling of O-GlcNAc glycosylation. Methods Enzymol. 598, 101–135 (2018).
Krystkowiak, I. & Davey, N. E. SLiMSearch: a framework for proteome-wide discovery and annotation of functional modules in intrinsically disordered regions. Nucleic Acids Res. 45, W464–W469 (2017).
Wulff-Fuentes, E. et al. The human O-GlcNAcome database and meta-analysis. Sci. Data 8, 25 (2021).
Huang, D. W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 4, 44–57 (2009).
Sherman, B. T. et al. DAVID: a web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 50, W216–W221 (2022).
Hornbeck, P. V. et al. PhosphoSitePlus, 2014: mutations, PTMs and recalibrations. Nucleic Acids Res. 43, D512–D520 (2015).
Oughtred, R. et al. The BioGRID database: a comprehensive biomedical resource of curated protein, genetic, and chemical interactions. Protein Sci. 30, 187–200 (2021).
Ramirez, D. H., Yang, B., D’Souza, A. K., Shen, D. & Woo, C. M. Truncation of the TPR domain of OGT alters substrate and glycosite selection. Anal. Bioanal. Chem. 413, 7385–7399 (2021).
Hu, C.-W. et al. Electrophilic probes for deciphering substrate recognition by O-GlcNAc transferase. Nat. Chem. Biol. 13, 1267–1273 (2017).
Bacigalupa, Z. A., Bhadiadra, C. H. & Reginato, M. J. O-GlcNAcylation: key regulator of glycolytic pathways. J. Bioenerg. Biomembr. 50, 189–198 (2018).
Liberti, M. V. & Locasale, J. W. The Warburg effect: how does it benefit cancer cells? Trends Biochem. Sci. 41, 211–218 (2016).
Shafi, R. et al. The O-GlcNAc transferase gene resides on the X chromosome and is essential for embryonic stem cell viability and mouse ontogeny. Proc. Natl Acad. Sci. USA 97, 5735–5739 (2000).
Forsythe, M. E. et al. Caenorhabditis elegans ortholog of a diabetes susceptibility locus: oga-1 (O-GlcNAcase) knockout impacts O-GlcNAc cycling, metabolism, and dauer. Proc. Natl Acad. Sci. USA 103, 11952–11957 (2006).
Alteen, M. G. et al. Potent de novo macrocyclic peptides that inhibit O-GlcNAc transferase through an allosteric mechanism. Angew. Chem. Int. Ed. 62, e202215671 (2023).
Alteen, M. G. et al. Phage display uncovers a sequence motif that drives polypeptide binding to a conserved regulatory exosite of O-GlcNAc transferase. Preprint at bioRxiv https://doi.org/10.1101/2023.03.15.532872 (2023).
Vonrhein, C. et al. Data processing and analysis with the autoPROC toolbox. Acta Crystallogr. D Biol. Crystallogr. 67, 293–302 (2011).
Evans, P. R. & Murshudov, G. N. How good are my data and what is the resolution? Acta Crystallogr. D Biol. Crystallogr. 69, 1204–1214 (2013).
Evans, P. Scaling and assessment of data quality. Acta Crystallogr. D Biol. Crystallogr. 62, 72–82 (2006).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Adams, P. D. et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr. D Biol. Crystallogr. 58, 1948–1954 (2002).
Huttlin, E. L. et al. Dual proteome-scale networks reveal cell-specific remodeling of the human interactome. Cell 184, 3022–3040.e28 (2021).
Sievers, F. et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011).
Waterhouse, A. M., Procter, J. B., Martin, D. M. A., Clamp, M. & Barton, G. J. Jalview Version 2—a multiple sequence alignment editor and analysis workbench. Bioinformatics 25, 1189–1191 (2009).
Yariv, B. et al. Using evolutionary data to make sense of macromolecules with a ‘face-lifted’ ConSurf. Protein Sci. 32, e4582 (2023).
Acknowledgements
We would like to thank members of the Jiang laboratory for insightful discussions and suggestions. This research was funded by National Institutes of Health (NIH) grant R01 GM121718 (J.J.), the University of Wisconsin-Madison Vilas Faculty Early Career Investigator Award (J.J.) and the National Science Foundation Graduate Research Fellowship under grant DGE-1747503 (C.M.B.). We also thank K. Satyshur for helpful suggestions on structure refinement; staff members of the Life Sciences Collaborative Access Team for assisting with X-ray data collection; and the University of Wisconsin Carbone Cancer Center Small Molecule Screening Facility and Drug Development Core Medicinal Chemistry Center (supported by NIH P30 CA014520) for use of the facilities and services. The authors acknowledge support from the National Genomics Infrastructure in Stockholm, funded by Science for Life Laboratory, the Knut and Alice Wallenberg Foundation and the Swedish Research Council, and the SNIC/Uppsala Multidisciplinary Center for Advanced Computational Science for assistance with massively parallel sequencing and access to the UPPMAX computational infrastructure. We thank N. E. Davey and L. Simonetti for support with the computational pipeline for next-generation sequencing data analysis.
Author information
Authors and Affiliations
Contributions
C.M.B., J.X. and J.J. conceptualized the project. J.J. oversaw all aspects of experiments and manuscript preparation. C.M.B. and J.X. purified proteins and performed thermal shift assays. C.M.B. performed fluorescence polarization and bioinformatic analysis and carried out all cellular experiments. J.X. crystallized, structurally determined and computationally analyzed protein–peptide complexes and performed microscale thermophoresis analysis. C.B. performed phage display screening, next-generation sequencing and peptide motif analysis, with supervision by Y.I. A.W. measured the enzymatic activities of OGT variants. C.M.B., J.X. and J.J. wrote the manuscript. All authors participated in editing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Charlie Fehl, Ramon Hurtado-Guerrero, Matthew Pratt and the other, anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
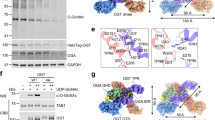
Extended Data Fig. 1 OGT binding evaluation of motif-containing peptides.
(a) Sequences of one consensus (CP37) and seven natural motif-containing peptides along with their apparent binding affinities (EC50) from competitive fluorescence polarization assay using 5-FAM-SMG9 peptide and OGT4.5. Standard deviations from three biological replicates are shown. PxYx[I/L] motif in each peptide is highlighted in red. (b) Thermal shift assay showing the shift in denaturation temperature (Tm) of OGT and OGT4.5 with peptide incubation, n = 3. (c) Competitive fluorescence polarization assay between 5-FAM-SMG9 and motif-containing peptides AK9 (blue), DENND1B (green), OR2AG1 (purple), JMJD1C (brown), and CAMKMT (cyan) for OGT4.5 binding. Data are presented as individual measurements ± SD of mean of three biological replicates.
Extended Data Fig. 2
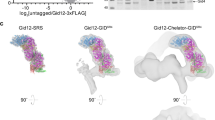
(a) Zoom-in view at the Int-D binding site demonstrating hydrophobic interactions between ZNF831 peptide (shown as sticks) and Int-D in OGT4.5 (shown as surface). Red and white colors represent the least and most hydrophobic areas, respectively. (b) Zoom-in view at the Int-D binding site demonstrating polar interactions between ZNF831 peptide (shown as purple sticks) and Int-D in OGT4.5 (shown as surface) with interacting OGT residues shown in sticks. 2Fo–Fc electron density map of ZNF831 peptide is shown as grey mesh and contoured at 1.0 σ, Fo–Fc electron density map is shown as green mesh and contoured at 3.0 σ. (c) Zoom-in view at the Int-D binding site demonstrating hydrophobic interactions between CP37 peptide (shown as sticks) and Int-D in OGT4.5 (shown as surface). Red and white colors represent the least and most hydrophobic areas, respectively. (d) Zoom-in view at the Int-D binding site demonstrating polar interactions between CP37 peptide (shown as blue sticks) and Int-D in OGT4.5 (shown as surface) with interacting OGT residues shown in sticks. 2Fo–Fc electron density map of CP37 peptide is shown as grey mesh and contoured at 1.0 σ, Fo–Fc electron density map is shown as green mesh and contoured at 3.0 σ. (e) Superimposition of OGT4.5:UDP-GlcNAc (4GZ5, grey), OGT4.5:UDP-GlcNAc:SMG9 (magenta), OGT4.5:UDP-GlcNAc:ZNF831 (purple), and OGT4.5:UDP-GlcNAc:CP37 (blue) crystal structures showing peptide binding to Int-D does not change the overall structure of OGT4.5. (f) A proposed model of a substrate peptide (shown as brown cartoon) binding to OGT4.5 (shown as surface, domains colored as in Fig. 1a). Int-D and TPR domain interactions facilitate substate glycosylation in the active site of OGT. G symbol represents GlcNAc moiety.
Extended Data Fig. 3 Evolutionary conservation analyses of OGT intervening domain.
(a) Sequences of 50 metazoan OGT homologs, including 25 homologs from vertebrates (highlighted in black box), were aligned by Clustal Omega58. Jalview59 was used to review the alignment results and generate the figure. Residues are colored according to the percentage identity in each column (>80 %, dark blue; > 60 %, blue; > 40%, light blue; < 40%, white). Int-D residues involved in motif interaction are highlighted in red boxes. (b) ConSurf60 analysis of metazoan (left) and vertebrate (right) OGT evolutionary conservation profile. Residues on the Int-D binding site are conserved among vertebrates but not invertebrates. Residues are colored from green to purple by variability across the aligned structures.
Extended Data Fig. 4 OGT Int-D mutants reduce peptide binding but retain intrinsic activity.
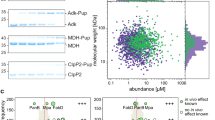
(a) Thermal shift assay showing the mutation induced change in OGT denaturation temperature (Tm). Data are presented as individual measurements ± SD of mean of three biological replicates. (b) Saturation fluorescence polarization (FP) binding assay of fluorescently labeled 5-FAM-SMG9 peptide with WT OGT (black) and mutants F723R (orange), F723E (blue), and I787R (green). Data are presented as mean values ± SD of three biological replicates. (c) Radiolabeled activity assay measuring integration of UDP-3H-GlcNAc onto CKII peptide by recombinantly purified WT OGT (WT) or OGT-N791A mutant (N791A). Data are presented as individual measurements ± SD of mean of three biological replicates.
Extended Data Fig. 5 The Int-D binding site is a motif-dependent regulator of protein association and O-GlcNAcylation.
Co-immunoprecipitation of cMyc-SMG9 with Flag-OGT (WT) or (a) Flag-OGT-I734R (I734R), (b) Flag-OGT-I787E (I787E), (c) Flag-OGT-I787E-N791A (2 M), from TRex-293 cells, followed by western blot. (d) Co-immunoprecipitation of endogenous OGA with Flag-OGT (W) or Flag-OGT-N791A (M) from TRex-293 cells, followed by western blot. (e) O-GlcNAcylation detection on CaMKMT from cells co-expressed with Flag-OGT (W) or Flag-OGT-N791A (M). HA-tagged CaMKMT was immunoprecipitated from TRex-293 cell lysate by anti-HA agarose and the O-GlcNAcylation was detected with the same GalT assay as SMG9. (f) O-GlcNAcylation detection on Flag-OGT (W), Flag-OGT-N791A (M), and Flag-HA-OGA-D175N. Flag-tagged OGT and OGA were immunoprecipitated from TRex-293 cell lysate by Flag-agarose, followed by western blot. All whole cell lysates have endogenous OGT knocked down. Blots are representative of at least three biological replicates.
Extended Data Fig. 6 Global O-GlcNAcylation is unperturbed by Int-D site mutation and single nutrient deprivation.
(a) Western blot detection of global O-GlcNAcylation in TRex-293 cells overexpressing OGT (WT), OGT-I787E (I787E), or OGT-I734R (I734R) with endogenous OGT knockdown (shOGT). Western blots of O-GlcNAcylation under high (H) and low (L) glucose (b) or serum (c) treatment with OGT (W) or OGT-N791A (M) overexpression. High nutrient conditions include DMEM with 4.5 g/L glucose and 10% FBS, low nutrient conditions include DMEM with 0.45 g/L glucose and 1% FBS. Blots are representative of at least three biological replicates.
Extended Data Fig. 7 Delayed response to nutrient deprivation in HeLa cells.
Western blot showing time course study of O-GlcNAcylation response to nutrient deprivation in HeLa cells stably expressing OGT (W) and OGT-N791A (M). Cells were induced for 48 hours prior to high or low nutrient treatment, then samples were collected at 3, 8, and 24 hours of treatment. Western blots are representative of three biological replicates.
Supplementary information
Supplementary Information
Supplementary Figs. 1 and 2, Tables 1 and 2 and Supplementary Fig. 2 source data.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Uncropped western blots.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Uncropped western blots.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Uncropped western blots.
Source Data Extended Data Fig. 6
Uncropped western blots.
Source Data Extended Data Fig. 7
Uncropped western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Blankenship, C.M., Xie, J., Benz, C. et al. Motif-dependent binding on the intervening domain regulates O-GlcNAc transferase. Nat Chem Biol 19, 1423–1431 (2023). https://doi.org/10.1038/s41589-023-01422-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-023-01422-2