Abstract
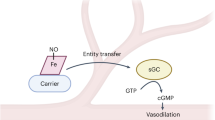
Nitric oxide (NO) is an endogenously produced signaling molecule that regulates blood flow and platelet activation. However, intracellular and intravascular diffusion of NO are limited by scavenging reactions with several hemoproteins, raising questions as to how free NO can signal in hemoprotein-rich environments. We explore the hypothesis that NO can be stabilized as a labile ferrous heme–nitrosyl complex (Fe2+-NO, NO-ferroheme). We observe a reaction between NO, labile ferric heme (Fe3+) and reduced thiols to yield NO-ferroheme and a thiyl radical. This thiol-catalyzed reductive nitrosylation occurs when heme is solubilized in lipophilic environments such as red blood cell membranes or bound to serum albumin. The resulting NO-ferroheme resists oxidative inactivation, is soluble in cell membranes and is transported intravascularly by albumin to promote potent vasodilation. We therefore provide an alternative route for NO delivery from erythrocytes and blood via transfer of NO-ferroheme and activation of apo-soluble guanylyl cyclase.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All the data generated in this study are available within the main text and the Supplementary Information file. Data are also available from the corresponding author upon request. The source datasets generated and analyzed during the current study are available on Dryad at https://doi.org/10.5061/dryad.mw6m9062d. Source data are provided with this paper.
References
Chambers, I. G., Willoughby, M. M., Hamza, I. & Reddi, A. R. One ring to bring them all and in the darkness bind them: the trafficking of heme without deliverers. Biochim. Biophys. Acta 1868, 118881 (2021).
Sun, F. et al. HRG-9 homologues regulate haem trafficking from haem-enriched compartments. Nature 610, 768–774 (2022).
Hanna, D. A. et al. Heme dynamics and trafficking factors revealed by genetically encoded fluorescent heme sensors. Proc. Natl Acad. Sci. USA 113, 7539–7544 (2016).
Dai, Y., Sweeny, E. A., Schlanger, S., Ghosh, A. & Stuehr, D. J. GAPDH delivers heme to soluble guanylyl cyclase. J. Biol. Chem. 295, 8145–8154 (2020).
Kharitonov, V. G., Sharma, V. S., Magde, D. & Koesling, D. Kinetics of nitric oxide dissociation from five- and six-coordinate nitrosyl hemes and heme proteins, including soluble guanylate cyclase. Biochemistry 36, 6814–6818 (1997).
Ford, P. C. & Miranda, K. M. The solution chemistry of nitric oxide and other reactive nitrogen species. Nitric Oxide 103, 31–46 (2020).
Cosby, K. et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nat. Med. 9, 1498–1505 (2003).
Huang, Z. et al. Enzymatic function of hemoglobin as a nitrite reductase that produces NO under allosteric control. J. Clin. Invest. 115, 2099–2107 (2005).
Cortese-Krott, M. M. & Kelm, M. Endothelial nitric oxide synthase in red blood cells: key to a new erythrocrine function? Redox Biol. 2, 251–258 (2014).
Leo, F. et al. Red blood cell and endothelial eNOS independently regulate circulating nitric oxide metabolites and blood pressure. Circulation 144, 870–889 (2021).
Eich, R. F. et al. Mechanism of NO-induced oxidation of myoglobin and hemoglobin. Biochemistry 35, 6976–6983 (1996).
Straub, A. C. et al. Endothelial cell expression of haemoglobin α regulates nitric oxide signalling. Nature 491, 473–477 (2012).
Dejam, A. et al. Nitrite infusion in humans and nonhuman primates. Circulation 116, 1821–1831 (2007).
Chen, K., Piknova, B., Pittman, R. N., Schechter, A. N. & Popel, A. S. Nitric oxide from nitrite reduction by hemoglobin in the plasma and erythrocytes. Nitric Oxide 18, 47–60 (2008).
Lancaster, J. R. Simulation of the diffusion and reaction of endogenously produced nitric oxide. Proc. Natl Acad. Sci. USA 91, 8137–8141 (1994).
Liu, X. et al. Diffusion-limited reaction of free nitric oxide with erythrocytes. J. Biol. Chem. 273, 18709–18713 (1998).
Rodriguez, J., Maloney, R. E., Rassaf, T., Bryan, N. S. & Feelisch, M. Chemical nature of nitric oxide storage forms in rat vascular tissue. Proc. Natl Acad. Sci. USA 100, 336–341 (2003).
Bryan, N. S. et al. Cellular targets and mechanisms of nitros(yl)ation: an insight into their nature and kinetics in vivo. Proc. Natl Acad. Sci. USA 101, 4308–4313 (2004).
Nikitovic, D. & Holmgren, A. S-nitrosoglutathione is cleaved by the thioredoxin system with liberation of glutathione and redox regulating nitric oxide. J. Biol. Chem. 271, 19180–19185 (1996).
Basu, S. et al. Catalytic generation of N2O3 by the concerted nitrite reductase and anhydrase activity of hemoglobin. Nat. Chem. Biol. 3, 785–794 (2007).
Liu, Y., Buerk, D. G., Barbee, K. A. & Jaron, D. A mathematical model for the role of N2O3 in enhancing nitric oxide bioavailability following nitrite infusion. Nitric Oxide 60, 1–9 (2016).
Hopmann, K. H., Cardey, B., Gladwin, M. T., Kim-Shapiro, D. B. & Ghosh, A. Hemoglobin as a nitrite anhydrase: modeling methemoglobin-mediated N2O3 formation. Chem. Eur. J. 17, 6348–6358 (2011).
Ford, P. C. Reactions of NO and nitrite with heme models and proteins. Inorg. Chem. 49, 6226–6239 (2010).
Fernandez, B. O. & Ford, P. C. Nitrite catalyzes ferriheme protein reductive nitrosylation. J. Am. Chem. Soc. 125, 10510–10511 (2003).
Kleschyov, A. L. The NO-heme signaling hypothesis. Free Radic. Biol. Med. 112, 544–552 (2017).
Cannon, R. O. et al. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. J. Clin. Invest. 108, 279–287 (2001).
Sweeny, E. A. et al. Nitric oxide and heme-NO stimulate superoxide production by NADPH oxidase 5. Free Radic. Biol. Med. 172, 252–263 (2021).
Ignarro, L. J., Adams, J. B., Horwitz, P. M. & Wood, K. S. Activation of soluble guanylate cyclase by NO-hemoproteins involves NO-heme exchange. Comparison of heme-containing and heme-deficient enzyme forms. J. Biol. Chem. 261, 4997–5002 (1986).
Shimizu, T., Lengalova, A., Martínek, V. & Martínková, M. Heme: emergent roles of heme in signal transduction, functional regulation and as catalytic centres. Chem. Soc. Rev. 48, 5624–5657 (2019).
Wegiel, B., Hauser, C. J. & Otterbein, L. E. Heme as a danger molecule in pathogen recognition. Free Radic. Biol. Med. 89, 651–661 (2015).
Canesin, G., Hejazi, S. M., Swanson, K. D. & Wegiel, B. Heme-derived metabolic signals dictate immune responses. Front. Immunol. 11, 66 (2020).
Cao, C. & Fleming, M. D. The ins and outs of erythroid heme transport. Haematologica 100, 703 (2015).
Gladwin, M. T. et al. Relative role of heme nitrosylation and β-cysteine 93 nitrosation in the transport and metabolism of nitric oxide by hemoglobin in the human circulation. Proc. Natl Acad. Sci. USA 97, 9943–9948 (2000).
Erzurum, S. C. et al. Higher blood flow and circulating NO products offset high-altitude hypoxia among Tibetans. Proc. Natl Acad. Sci. USA 104, 17593–17598 (2007).
O’Keeffe, R. et al. Glutathione and the intracellular labile heme pool. Biometals 34, 221–228 (2021).
Cooper, C. E. Nitric oxide and iron proteins. Biochim. Biophys. Acta 1411, 290–309 (1999).
Hoshino, M., Maeda, M., Konishi, R., Seki, H. & Ford, P. C. Studies on the reaction mechanism for reductive nitrosylation of ferrihemoproteins in buffer solutions. J. Am. Chem. Soc. 118, 5702–5707 (1996).
Heinecke, J. L. et al. Nitrite reduction mediated by heme models. Routes to NO and HNO? J. Am. Chem. Soc. 135, 4007–4017 (2013).
MacArthur, P. H., Shiva, S. & Gladwin, M. T. Measurement of circulating nitrite and S-nitrosothiols by reductive chemiluminescence. J. Chromatogr. B 851, 93–105 (2007).
Basu, S., Wang, X., Galdwin, M. T. & Kim-Shapiro, D. B. Chemiluminescent detection of S‐nitrosated proteins: comparison of tri‐iodide, copper/CO/cysteine, and modified copper/cysteine methods. Methods Enzymol. 440, 137–156 (2008).
Singh, S. P., Wishnok, J. S., Keshive, M., Deen, W. M. & Tannenbaum, S. R. The chemistry of the S-nitrosoglutathione/glutathione system. Proc. Natl Acad. Sci. USA 93, 14428–14433 (1996).
Pou, S., Keaton, L., Surichamorn, W., Frigillana, P. & Rosen, G. M. Can nitric oxide be spin trapped by nitrone and nitroso compounds? Biochim. Biophys. Acta 1201, 118–124 (1994).
Chiabrando, D., Vinchi, F., Fiorito, V., Mercurio, S. & Tolosano, E. Heme in pathophysiology: a matter of scavenging, metabolism and trafficking across cell membranes. Front Pharm. 5, 61 (2014).
Salgado, M. T., Cao, Z., Nagababu, E., Mohanty, J. G. & Rifkind, J. M. Red blood cell membrane-facilitated release of nitrite-derived nitric oxide bioactivity. Biochemistry 54, 6712–6723 (2015).
Praneeth, V. K. K., Haupt, E. & Lehnert, N. Thiolate coordination to Fe(II)–porphyrin NO centers. J. Inorg. Biochem. 99, 940–948 (2005).
Goodrich, L. E., Paulat, F., Praneeth, V. K. K. & Lehnert, N. Electronic structure of heme-nitrosyls and its significance for nitric oxide reactivity, sensing, transport, and toxicity in biological systems. Inorg. Chem. 49, 6293–6316 (2010).
Witting, P. K., Douglas, D. J. & Mauk, A. G. Reaction of human myoglobin and H2O2: involvement of a thiyl radical produced at cysTEINE 110. J. Biol. Chem. 275, 20391–20398 (2000).
Wardell, M. et al. The atomic structure of human methemalbumin at 1.9 Å. Biochem. Biophys. Res. Commun. 291, 813–819 (2002).
Ascenzi, P., di Masi, A., Fanali, G. & Fasano, M. Heme-based catalytic properties of human serum albumin. Cell Death Discov. 1, 15025 (2015).
Hanson, M. S. et al. Methaemalbumin formation in sickle cell disease: effect on oxidative protein modification and HO-1 induction. Br. J. Haematol. 154, 502–511 (2011).
Carter, D. C. & Ho, J. X. in Advances in Protein Chemistry (eds. Anfinsen, C. B., Edsall, J. T., Richards, F. M. & Eisenberg, D. S.) vol. 45 153–203 (Academic, 1994).
Wang, B. et al. Nitrosyl myoglobins and their nitrite precursors: crystal structural and quantum mechanics and molecular mechanics theoretical investigations of preferred Fe–NO ligand orientations in myoglobin distal pockets. Biochemistry 57, 4788–4802 (2018).
Doyle, M. P. & Hoekstra, J. W. Oxidation of nitrogen oxides by bound dioxygen in hemoproteins. J. Inorg. Biochem. 14, 351–358 (1981).
Andersen, H. J. & Skibsted, L. H. Kinetics and mechanism of thermal oxidation and photooxidation of nitrosylmyoglobin in aqueous solution. J. Agric. Food Chem. 40, 1741–1750 (1992).
Naseem, K. M. & Roberts, W. Nitric oxide at a glance. Platelets 22, 148–152 (2011).
Stasch, J.-P. et al. Targeting the heme-oxidized nitric oxide receptor for selective vasodilatation of diseased blood vessels. J. Clin. Invest. 116, 2552–2561 (2006).
Becker-Pelster, E. M. et al. Inhaled mosliciguat (BAY 1237592): targeting pulmonary vasculature via activating apo-sGC. Respir. Res. 23, 272 (2022).
Wajih, N. et al. Erythrocytic bioactivation of nitrite and its potentiation by far-red light. Redox Biol. 20, 442–450 (2019).
Wajih, N. et al. The role of red blood cell S-nitrosation in nitrite bioactivation and its modulation by leucine and glucose. Redox Biol. 8, 415–421 (2016).
Shah, C. M., Bell, S. E., Locke, I. C., Chowdrey, H. S. & Gordge, M. P. Interactions between cell surface protein disulphide isomerase and S-nitrosoglutathione during nitric oxide delivery. Nitric Oxide 16, 135–142 (2007).
Mullershausen, F. et al. Rapid nitric oxide–induced desensitization of the cGMP response is caused by increased activity of phosphodiesterase type 5 paralleled by phosphorylation of the enzyme. J. Cell Biol. 155, 271–278 (2001).
Mingone, C. J., Gupte, S. A., Iesaki, T. & Wolin, M. S. Hypoxia enhances a cGMP-independent nitric oxide relaxing mechanism in pulmonary arteries. Am. J. Physiol. Lung Cell. Mol. Physiol. 285, L296–L304 (2003).
Nagababu, E. Ferriheme catalyzes nitric oxide reaction with glutathione to form S-nitrosoglutathione: a novel mechanism for formation of S-nitrosothiols. Free Radic. Biol. Med. 101, 296–304 (2016).
Reichenbach, G., Sabatini, S., Palombari, R. & Palmerini, C. A. Reaction mechanism between nitric oxide and glutathione mediated by Fe(III) myoglobin. Nitric Oxide 5, 395–401 (2001).
DeMartino, A. W., Kim-Shapiro, D. B., Patel, R. P. & Gladwin, M. T. Nitrite and nitrate chemical biology and signalling. Br. J. Pharmacol. 176, 228–245 (2019).
Dent, M. R., DeMartino, A. W., Tejero, J. & Gladwin, M. T. Endogenous hemoprotein-dependent signaling pathways of nitric oxide and nitrite. Inorg. Chem. 60, 15918–15940 (2021).
Parent, M. et al. In situ microparticles loaded with S-nitrosoglutathione protect from stroke. PLoS One 10, e0144659 (2015).
van ‘t Erve, T. J., Wagner, B. A., Ryckman, K. K., Raife, T. J. & Buettner, G. R. The concentration of glutathione in human erythrocytes is a Heritable Trait. Free Radic. Biol. Med. https://doi.org/10.1016/j.freeradbiomed.2013.08.002 (2013).
Donegan, R. K., Moore, C. M., Hanna, D. A. & Reddi, A. R. Handling heme: the mechanisms underlying the movement of heme within and between cells. Free Radic. Biol. Med. 133, 88–100 (2019).
Krishnamurthy, P. & Schuetz, J. D. The ABC transporter Abcg2/Bcrp: role in hypoxia mediated survival. Biometals 18, 349–358 (2005).
Ascenzi, P., di Masi, A., De Sanctis, G., Coletta, M. & Fasano, M. Ibuprofen modulates allosterically NO dissociation from ferrous nitrosylated human serum heme-albumin by binding to three sites. Biochem. Biophys. Res. Commun. 387, 83–86 (2009).
Ascenzi, P., Imperi, F., Coletta, M. & Fasano, M. Abacavir and warfarin modulate allosterically kinetics of NO dissociation from ferrous nitrosylated human serum heme-albumin. Biochem. Biophys. Res. Commun. 369, 686–691 (2008).
Jennifer, B. et al. Transferrin receptor 1 is a cellular receptor for human heme-albumin. Commun. Biol. 3, 1–13 (2020).
Shvartsman, M., Bilican, S. & Lancrin, C. Iron deficiency disrupts embryonic haematopoiesis but not the endothelial to haematopoietic transition. Sci. Rep. 9, 6414 (2019).
Wu, S. M. et al. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell 127, 1137–1150 (2006).
Vogel, S. M., Minshall, R. D., Pilipović, M., Tiruppathi, C. & Malik, A. B. Albumin uptake and transcytosis in endothelial cells in vivo induced by albumin-binding protein. Am. J. Physiol. Lung Cell. Mol. Physiol. 281, L1512–L1522 (2001).
Hart, T. W. Some observations concerning the S-nitroso and S-phenylsulphonyl derivatives of l-cysteine and glutathione. Tetrahedron Lett. 26, 2013–2016 (1985).
Zijlstra, W. G., Buursma, A & van Assendelft, O.W. Visible and Near Infrared Absorption Spectra of Human and Animal Haemoglobin Determination and Application (CRC, 2021).
Maragos, C. M. et al. Complexes of NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant effects. J. Med. Chem. 34, 3242–3247 (1991).
Hughan, K. S. et al. Conjugated linoleic acid modulates clinical responses to oral nitrite and nitrate. Hypertension 70, 634–644 (2017).
Stoll, S. & Schweiger, A. EasySpin, a comprehensive software package for spectral simulation and analysis in EPR. J. Magn. Reson. 178, 42–55 (2006).
Wajih, N. et al. Potential therapeutic action of nitrite in sickle cell disease. Redox Biol. 12, 1026–1039 (2017).
Acknowledgements
We thank M. Guthold and H. Lee for help with DIC microscopy. This work was supported by NIH grants R01 HL125886 (J.T. and M.T.G.), R01 HL098032 (M.T.G. and D.B.K.-S.), K08 HL136857 (J.J.R.) and DOD grant W81XWH2210198 (J.T. and J.J.R.).
Author information
Authors and Affiliations
Contributions
A.W.D. designed and performed experiments, analyzed data and wrote the initial and final drafts of the paper. L.P. performed and helped design experiments, including the initial ones discovering the thiol-based catalysis, and analyzed data. M.R.D., X.C., Q.X., B.S.G., J.T., S.B., E.A., J.J.R. and Y.J. also performed experiments and/or analyzed data. All authors reviewed and edited the manuscript. M.T.G. and D.B.K.-S. directed the research and designed experiments, interpreted data and share senior authorship.
Corresponding authors
Ethics declarations
Competing interests
A.W.D., J.J.R., M.T.G., J.T., M.R.D., D.B.K.-S. and L.P. have a provisional patent filed at the University of Pittsburgh (application no. 63/420,030), related to the creation and use of NO-ferroheme. Though not related directly to NO-ferroheme, A.W.D., J.T., J.J.R., M.T.G., M.R.D. and Q.X. are co-inventors on patents and patent applications directed at the use of heme proteins as therapeutic agents. M.T.G., D.B.K.-S. and J.J.R. are co-inventors on patent and/or patent applications related to sodium nitrite as a therapeutic. Some of these patents are licensed to Globin Solutions, Inc. J.J.R., M.T.G. and J.T. are shareholders of Globin Solutions. J.J.R. and J.T. are officers and directors of Globin Solutions. A.W.D. is a consultant of Globin Solutions. M.T.G. is a consultant, director and scientific advisor to Globin Solutions. The other authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Arnab Ghosh, Emil Martin and Douglas Thomas for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Scheme depicting electron transfer to generate NO-ferroheme.
Ferric labile heme reacts with NO to generate a labile nitrosyl ferric heme, which readily is reduced by 1 electron by a small thiol like glutathione (GSH). The mechanism of transfer may follow an inner sphere mechanism (I.S.) where the GSH binds the nitrosyl ferric heme first then transfers the electron, or an outer sphere mechanism (O.S) where binding does not occur. In the presence of excess NO, the generated thiyl radical from either mechanism will react with NO to yield S-nitrosoglutathione (GSNO).
Supplementary information
Supplementary Information
Supplementary Figs. 1–7.
Supplementary Data 1
Data for Supplementary Fig. 1. Example NO traces from NO chemiluminescence analyzer, source data.
Supplementary Data 2
Data for Supplementary Fig. 2. Formation of NO-ferroheme via GSH-catalyzed reductive nitrosylation of ferric heme in albumin at different NO concentrations, source data.
Supplementary Data 3
Data for Supplementary Fig. 3. Other transfers of NO-ferroheme, source data.
Supplementary Data 4
Data for Supplementary Fig. 4. NO-ferroheme albumin stability in the presence of an equivalent of oxyhemoglobin, source data.
Supplementary Data 5
Data for Supplementary Fig. 5. Other relevant platelet control experiments, source data.
Supplementary Data 6
Data for Supplementary Fig. 6. Elimination of nitrite as potential vasodilating species during administration of NO-ferroheme albumin solution, source data.
Supplementary Data 7
Data for Supplementary Fig. 7. Typified flow cytometry gating for platelet sorting and platelet activation determination.
Source data
Source Data Fig. 1
Data for Fig. 1.
Source Data Fig. 2
Data for Fig. 2.
Source Data Fig. 3
Data for Fig. 3.
Source Data Fig. 4
Data for Fig. 4.
Source Data Fig. 5
Data for Fig. 5.
Source Data Fig. 6
Data for Fig. 6.
Source Data Extended Data Fig. 1
Chemdraw file for Extended Data Fig. 1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
DeMartino, A.W., Poudel, L., Dent, M.R. et al. Thiol-catalyzed formation of NO-ferroheme regulates intravascular NO signaling. Nat Chem Biol 19, 1256–1266 (2023). https://doi.org/10.1038/s41589-023-01413-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-023-01413-3
This article is cited by
-
Antibacterial mouthwash alters gut microbiome, reducing nutrient absorption and fat accumulation in Western diet-fed mice
Scientific Reports (2024)
-
NO signal
Nature Chemical Biology (2023)