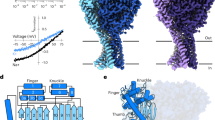
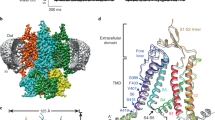
Abstract
Phe-Met-Arg-Phe-amide (FMRFamide)-activated sodium channels (FaNaCs) are a family of channels activated by the neuropeptide FMRFamide, and, to date, the underlying ligand gating mechanism remains unknown. Here we present the high-resolution cryo-electron microscopy structures of Aplysia californica FaNaC in both apo and FMRFamide-bound states. AcFaNaC forms a chalice-shaped trimer and possesses several notable features, including two FaNaC-specific insertion regions, a distinct finger domain and non-domain-swapped transmembrane helix 2 in the transmembrane domain (TMD). One FMRFamide binds to each subunit in a cleft located in the top-most region of the extracellular domain, with participation of residues from the neighboring subunit. Bound FMRFamide adopts an extended conformation. FMRFamide binds tightly to A. californica FaNaC in an N terminus-in manner, which causes collapse of the binding cleft and induces large local conformational rearrangements. Such conformational changes are propagated downward toward the TMD via the palm domain, possibly resulting in outward movement of the TMD and dilation of the ion conduction pore.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Structure coordinates and cryo-electron microscopy density maps have been deposited at the PDB and Electron Microscopy Data Bank under accession numbers 7YVC and EMD-34123 for AcFaNaC in the apo state and 7YVB and EMD-34122 for AcFaNaC in the FMRFamide-bound state. Other structure coordinates analyzed in the paper can be downloaded from PDB under accession numbers 4NTY, 6AVE and 4FZ0 (Gallus gallus ASIC1). Source data are provided with this paper.
References
Grimmelikhuijzen, C. J. & Hauser, F. Mini-review: the evolution of neuropeptide signaling. Regul. Pept. 177 Suppl, S6–S9 (2012).
Frontali, N., Williams, L. & Welsh, J. H. Heart excitatory and inhibitory substances in molluscan ganglia. Comp. Biochem. Physiol. 22, 833–841 (1967).
Price, D. A. & Greenberg, M. J. Structure of a molluscan cardioexcitatory neuropeptide. Science 197, 670–671 (1977).
Walker, R. J., Papaioannou, S. & Holden-Dye, L. A review of FMRFamide- and RFamide-like peptides in metazoa. Invert. Neurosci. 9, 111–153 (2009).
Brain, S. D. & Cox, H. M. Neuropeptides and their receptors: innovative science providing novel therapeutic targets. Br. J. Pharmacol. 147 Suppl 1, S202–S211 (2006).
Golubovic, A. et al. A peptide-gated ion channel from the freshwater polyp Hydra. J. Biol. Chem. 282, 35098–35103 (2007).
Kellenberger, S. & Schild, L. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol. Rev. 82, 735–767 (2002).
Askwith, C. C. et al. Neuropeptide FF and FMRFamide potentiate acid-evoked currents from sensory neurons and proton-gated DEG/ENaC channels. Neuron 26, 133–141 (2000).
Lingueglia, E., Champigny, G., Lazdunski, M. & Barbry, P. Cloning of the amiloride-sensitive FMRFamide peptide-gated sodium channel. Nature 378, 730–733 (1995).
Furukawa, Y., Miyawaki, Y. & Abe, G. Molecular cloning and functional characterization of the Aplysia FMRFamide-gated Na+ channel. Pflug. Arch. 451, 646–656 (2006).
Jeziorski, M. C., Green, K. A., Sommerville, J. & Cottrell, G. A. Cloning and expression of a FMRFamide-gated Na+ channel from Helisoma trivolvis and comparison with the native neuronal channel. J. Physiol. 526 Pt 1, 13–25 (2000).
Perry, S. J., Straub, V. A., Schofield, M. G., Burke, J. F. & Benjamin, P. R. Neuronal expression of an FMRFamide-gated Na+ channel and its modulation by acid pH. J. Neurosci. 21, 5559–5567 (2001).
Dandamudi, M., Hausen, H. & Lynagh, T. Comparative analysis defines a broader FMRFamide-gated sodium channel family and determinants of neuropeptide sensitivity. J. Biol. Chem. 298, 102086 (2022).
Cottrell, G. A., Jeziorski, M. C. & Green, K. A. Location of a ligand recognition site of FMRFamide-gated Na+ channels. FEBS Lett. 489, 71–74 (2001).
Cottrell, G. A. Domain near TM1 influences agonist and antagonist responses of peptide-gated Na+ channels. Pflug. Arch. 450, 168–177 (2005).
Niu, Y. Y. et al. Exploration of the peptide recognition of an amiloride-sensitive FMRFamide peptide-gated sodium channel. J. Biol. Chem. 291, 7571–7582 (2016).
Waldmann, R., Champigny, G. & Lazdunski, M. Functional degenerin-containing chimeras identify residues essential for amiloride-sensitive Na+ channel function. J. Biol. Chem. 270, 11735–11737 (1995).
Jasti, J., Furukawa, H., Gonzales, E. B. & Gouaux, E. Structure of acid-sensing ion channel 1 at 1.9 A resolution and low pH. Nature 449, 316–323 (2007).
Noreng, S., Bharadwaj, A., Posert, R., Yoshioka, C. & Baconguis, I. Structure of the human epithelial sodium channel by cryo-electron microscopy. eLife 7, e39340 (2018).
Yoder, N., Yoshioka, C. & Gouaux, E. Gating mechanisms of acid-sensing ion channels. Nature 555, 397–401 (2018).
Gonzales, E. B., Kawate, T. & Gouaux, E. Pore architecture and ion sites in acid-sensing ion channels and P2X receptors. Nature 460, 599–604 (2009).
Dawson, R. J. et al. Structure of the acid-sensing ion channel 1 in complex with the gating modifier psalmotoxin 1. Nat. Commun. 3, 936 (2012).
Baconguis, I., Bohlen, C. J., Goehring, A., Julius, D. & Gouaux, E. X-ray structure of acid-sensing ion channel 1–snake toxin complex reveals open state of a Na+-selective channel. Cell 156, 717–729 (2014).
Baconguis, I. & Gouaux, E. Structural plasticity and dynamic selectivity of acid-sensing ion channel-spider toxin complexes. Nature 489, 400–405 (2012).
Poet, M. et al. Exploration of the pore structure of a peptide-gated Na+ channel. EMBO J. 20, 5595–5602 (2001).
Yoder, N. & Gouaux, E. The His-Gly motif of acid-sensing ion channels resides in a reentrant ‘loop’ implicated in gating and ion selectivity. eLife 9, e56527 (2020).
Fujimoto, A., Kodani, Y. & Furukawa, Y. Modulation of the FMRFamide-gated Na+ channel by external Ca2+. Pflug. Arch. 469, 1335–1347 (2017).
Kodani, Y. & Furukawa, Y. Position 552 in a FMRFamide-gated Na+ channel affects the gating properties and the potency of FMRFamide. Zool. Sci. 27, 440–448 (2010).
Kodani, Y. & Furukawa, Y. Electrostatic charge at position 552 affects the activation and permeation of FMRFamide-gated Na+ channels. J. Physiol. Sci. 64, 141–150 (2014).
Lynagh, T. et al. A selectivity filter at the intracellular end of the acid-sensing ion channel pore. eLife 6, e24630 (2017).
Lynagh, T. et al. Determinants of ion selectivity in ASIC1a- and ASIC2a-containing acid-sensing ion channels. J. Gen. Physiol. 152, e201812297 (2020).
Chen, Z., Lin, S., Xie, T., Lin, J. M. & Canessa, C. M. A flexible GAS belt responds to pore mutations changing the ion selectivity of proton-gated channels. J. Gen. Physiol. 154, e202112978 (2022).
Green, K. A. & Cottrell, G. A. Block of the helix FMRFamide-gated Na+ channel by FMRFamide and its analogues. J. Physiol. 519 Pt 1, 47–56 (1999).
Cottrell, G. A. The first peptide-gated ion channel. J. Exp. Biol. 200, 2377–2386 (1997).
Plaxco, K. W., Simons, K. T. & Baker, D. Contact order, transition state placement and the refolding rates of single domain proteins. J. Mol. Biol. 277, 985–994 (1998).
Li, T., Yang, Y. & Canessa, C. M. Interaction of the aromatics Tyr-72/Trp-288 in the interface of the extracellular and transmembrane domains is essential for proton gating of acid-sensing ion channels. J. Biol. Chem. 284, 4689–4694 (2009).
Shi, S. et al. Base of the thumb domain modulates epithelial sodium channel gating. J. Biol. Chem. 286, 14753–14761 (2011).
Lynagh, T., Mikhaleva, Y., Colding, J. M., Glover, J. C. & Pless, S. A. Acid-sensing ion channels emerged over 600 Mya and are conserved throughout the deuterostomes. Proc. Natl Acad. Sci. USA 115, 8430–8435 (2018).
Rook, M. L., Musgaard, M. & MacLean, D. M. Coupling structure with function in acid-sensing ion channels: challenges in pursuit of proton sensors. J. Physiol. 599, 417–430 (2021).
Yu, Y. et al. A nonproton ligand sensor in the acid-sensing ion channel. Neuron 68, 61–72 (2010).
Yang, X. N. et al. The nonproton ligand of acid-sensing ion channel 3 activates mollusk-specific FaNaC channels via a mechanism independent of the native FMRFamide peptide. J. Biol. Chem. 292, 21662–21675 (2017).
Bargeton, B. et al. Mutations in the palm domain disrupt modulation of acid-sensing ion channel 1a currents by neuropeptides. Sci. Rep. 9, 2599 (2019).
Borg, C. B. et al. Mechanism and site of action of big dynorphin on ASIC1a. Proc. Natl Acad. Sci. USA 117, 7447–7454 (2020).
Leisle, L. et al. Dynorphin neuropeptides decrease apparent proton affinity of ASIC1a by occluding the acidic pocket. J. Med. Chem. 64, 13299–13311 (2021).
Schanuel, S. M., Bell, K. A., Henderson, S. C. & McQuiston, A. R. Heterologous expression of the invertebrate FMRFamide-gated sodium channel as a mechanism to selectively activate mammalian neurons. Neuroscience 155, 374–386 (2008).
DeLaney, K. et al. New techniques, applications and perspectives in neuropeptide research. J. Exp. Biol. 221, jeb151167 (2018).
Morales-Perez, C. L., Noviello, C. M. & Hibbs, R. E. Manipulation of subunit stoichiometry in heteromeric membrane proteins. Structure 24, 797–805 (2016).
Scheres, S. H. RELION: implementation of a Bayesian approach to cryo-EM structure determination. J. Struct. Biol. 180, 519–530 (2012).
Kucukelbir, A., Sigworth, F. J. & Tagare, H. D. Quantifying the local resolution of cryo-EM density maps. Nat. Methods 11, 63–65 (2014).
Zheng, S. Q. et al. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 14, 331–332 (2017).
Zhang, K. Gctf: real-time CTF determination and correction. J. Struct. Biol. 193, 1–12 (2016).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D 66, 486–501 (2010).
Afonine, P. V., Headd, J. J., Terwilliger, T. C. & Adams, P. D. New tool: phenix.real_space_refine. Computational Crystallogr. Newsl. 4, 43–44 (2013).
Moriarty, N. W., Grosse-Kunstleve, R. W. & Adams, P. D. Electronic ligand builder and optimization workbench (eLBOW): a tool for ligand coordinate and restraint generation. Acta Crystallogr. D 65, 1074–1080 (2009).
Chen, V. B. et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D 66, 12–21 (2010).
Smart, O. S., Neduvelil, J. G., Wang, X., Wallace, B. A. & Sansom, M. S. HOLE: a program for the analysis of the pore dimensions of ion channel structural models. J. Mol. Graph. 14, 354–360 (1996).
Wallace, A. C., Laskowski, R. A. & Thornton, J. M. LIGPLOT: a program to generate schematic diagrams of protein–ligand interactions. Protein Eng. 8, 127–134 (1995).
Schrodinger, LLC. The PyMOL Molecular Graphics System, Version 1.8 (2015).
Pettersen, E. F. et al. UCSF Chimera—a visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004).
Jo, S., Kim, T., Iyer, V. G. & Im, W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J. Comput. Chem. 29, 1859–1865 (2008).
Phillips, J. C. et al. Scalable molecular dynamics with NAMD. J. Comput. Chem. 26, 1781–1802 (2005).
Klauda, J. B. et al. Update of the CHARMM all-atom additive force field for lipids: validation on six lipid types. J. Phys. Chem. B 114, 7830–7843 (2010).
Acknowledgements
We thank Y. Jiang’s support for the initiation of this project. Single-particle cryo-electron microscopy data were collected at the Peking University Cryo-Electron Microscopy Facility. We thank the cryo-electron microscopy platform and the School of Life Sciences of Peking University for cryo-electron microscopy data collection. We are grateful to Z. Guo, G. Wang, X. Pei, C. Qin and N. Gao for their help with cryo-electron microscopy experiments. We thank the National Center for Protein Sciences at Peking University for other technical support. We thank J. Lin for sharing homemade antibodies to GFP. We thank Y. Bai for assistance in confocal imaging. This work was supported, in part, by grants from the Ministry of Science and Technology of China (2020YFA0908500 to S.Y. and Q.C. and 2021YFA1302300 to Z.Z.), the National Natural Science Foundation of China (32071202 and 32271012 to Q.C., 32171201 to Z.Z., 32071103 to Y.T. and 31971127 to S.Y.) and the Tianjin Fund for Distinguished Young Scholars (20JCJQJC00080 to Q.C.).
Author information
Authors and Affiliations
Contributions
Q.C., Y.T., S.Y. and Z.Z. conceived and supervised the project. F.L., H.F. and X. Z. prepared the samples. Y.D., Z.Z. and Q.C. performed data acquisition, image processing and structure determination. L.L. and Y.T. performed electrophysiology experiments. J.L., H.W., Y.T. and Q.C. performed MD simulations. All authors participated in research design, data analysis and paper preparation.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Eric Gouaux, Stephan Kellenberger, Stephan Pless, Changlin Tian and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Sequence alignment of FaNaCs and cASIC1.
Sequence alignment of AcFaNaC (accession number XP_012938736.1), AkFaNaC (accession number BAE07082.1), HtFaNaC (accession number AF80601.1), LsFaNaC (accession number AAK20896.1), HaFaNaC (accession number CAA63084.1), and cASIC1 (accession number AAY28986.1). Secondary structures are colored as in Fig. 1d and are indicated by cylinders (helices), arrows (β-strands), solid line (loop), and dashed line (disordered region). Conserved residues are highlighted in red. Purple and yellow crosses mark residues involved in FMRFamide binding from one subunit and the neighboring subunit, respectively. Green triangles mark previously proposed key residues important for peptide affinity differences. The purple triangle marks Phe 474 that shows a dramatic conformational change upon peptide binding. Blue circles mark residues that are potentially important for ion selectivity based on studies on ASIC1. Specific insertion I&II, the β6–β7-loop and the HG and GxS motifs are underlined and labeled. Multiple sequence alignment was performed using Clustal Omega (https://www.ebi.ac.uk/Tools/msa/clustalo/), and this figure is prepared using ESPript server (https://espript.ibcp.fr/ESPript/ESPript/).
Extended Data Fig. 2 Structure comparison of AcFaNaC and cASIC1.
a-d, Comparison of FMRFamdie-bound AcFaNaC (a) and low pH cASIC1-PcTx1 complex (pdb code 4FZ0) (b), and their superposition in front (c) and top (d) view. in a and b, the FMRFamide binding pocket in AcFaNaC is circled in purple, and potential proton sensing acidic pocket and His 73 in cASIC1 are circled in blue and green respectively. One FMRFamide is shown as sticks. Key secondary structural elements are labeled, with α2 highlighted in red. In b, PcTx1 is shown as gray surface. e-f, Comparison of TMD from AcFaNaC and cASIC1 in bottom (e) and front (f) view, with transmembrane helices labeled. In f, one subunit is removed for clarity and key residues are labeled. g, Superposition of FMRFamdie-bound AcFaNaC and low pH desensitized cASIC1 (pdb code 4NYK). h-i, Comparison of TMD from FMRFamdie-bound AcFaNaC and cASIC1 (pdb code 4NYK) in bottom (h) and front (i) view, with transmembrane helices labeled. In i, one subunit is removed for clarity and key residues are labeled.
Extended Data Fig. 3 Focused 3D classification of apo dataset on TMD.
Each class is shown in top (a), side (b) and bottom (c) views. The number of particles for each class is labeled at the bottom.
Extended Data Fig. 4 Solvation status of FMRFamide-bound AcFaNaC structure during MD simulations.
The snapshot of the MD simulation at t = 10 ns is shown. AcFaNaC is shown as cartoon, with each subunit colored differently. Lipids are shown as green lines, whereas water molecules are shown as red and white spheres. Inset, expanded view highlighting solvation of lateral fenestrations. Locations of lateral fenestrations are indicated by yellow dashed circle or arrows.
Extended Data Fig. 5 Location of TM1 residues that are accessible by sulfhydryl reagent.
Residues accessible in closed state are shown as yellow sticks, whereas residues accessible from intracellular side in open state are shown as blue sticks (equivalent residues in the original HaFaNaC are shown in parentheses in a). Trp 95, whose mutation to cysteine resulted in a nonfunctional channel, is shown as green sticks. Front (a) and top (b) views are shown.
Extended Data Fig. 6 Sample traces and concentration-response curves for WT and FMRFamide coordinating residues mutants of AcFaNaC.
Sample traces and concentration-response curve for each construct is boxed. For sample traces, increasing concentration of FMRFamide was applied by perfusion as indicated by bars. Concentration-response curves of mutant were measured using multiple independent cells (n = 7 (I147A), n = 6 (L168A), n = 5 (Q172A), n = 6 (N183A), n = 5 (F188A), n = 5 (T271A), n = 9 (Y272A), n = 8 (V274A), n = 7 (F453A)), and the curve for each mutant is shown as a solid line and is overlaid with that of WT, which is shown as dashed line. All data points are mean ± SEM.
Extended Data Fig. 7 Structural rearrangement around Phe 474 following FMRFamide binding.
a, Cartoon representation and density for regions around Phe 474 in AcFaNaC apo state at the level of 0.025 in the UCSF chimera. b, Cartoon representation and density for regions around Phe 474 in FMRFamide-bound AcFaNaC state at the level of 0.025 in the UCSF chimera.
Extended Data Fig. 8 Structural changes in the extracellular and central vestibules.
a, Front view, with one subunit removed for clarity. Residues that form constrictions with their sidechains (Val 123 and Tyr 542) or mainchain (Glu 124) in the junction between extracellular and central vestibules are shown as sticks. The distances between each pair of residues from two subunits are shown as dashed lines and labeled. b-d, Top view from central vestibule, with all three subunits shown, highlighting the changes in distances between Tyr 542 (b), Glu 124 (c), and Val 123 (d). Apo and FMRFamide bound structures are shown in pink and cyan respectively.
Extended Data Fig. 9 Confocal imaging, western blot, and sample traces of WT and selected mutants transiently expressed in HEK293 cells.
a, Confocal imaging. From left to right, three images in each box represent the indicated AcFaNaC variant fused with GFP, membrane marker KRas GTPase fused with mCherry, and merge, respectively. The scale bars represent 5 μm. The experiment was repeated twice independently with similar results. b, Western blotting. T stands for total extract after detergent solubilization, whereas S stands for supernatant after centrifugation. The experiment was repeated twice independently with similar results. c, Sample traces. FMRFamide (30 μM) was applied for each measurement as indicated by bars.
Extended Data Fig. 10 Location of biochemically identified residues important for substrate specificity and channel activity in FMRFamide bound structure.
a, An overview, with biochemically identified Asn 159, Met 179, and other related residues, such as residues involved in propagation of conformational changes from ECD to TMD (Phe 120 and Tyr 329) and residue key for ligand binding as well as initiation of activation (Phe 453) shown as sticks. b, Expanded view of residues around the FMRFamide binding pocket, with biochemically identified residues shown as purple or red sticks and their interacting partners shown as blue sticks. c, Expanded view of biochemically identified residues (green sticks) located in the palm domain.
Supplementary information
Supplementary Information
Supplementary Figs. 1–9 and Tables 1–5.
Supplementary Video 1
Zoomed-in view of the FMRFamide binding pocket. The finger domain and knuckle domain from the neighboring subunit in AcFaNaC are shown as cyan and marine cartoons, respectively. The FMRFamide molecule is shown as yellow sticks. Residues involved in the FMRFamide interaction are labeled. Hydrogen bonds are shown as dashed lines.
Supplementary Video 2
Morph between apo and FMRFamide-bound AcFaNaC structures around the ligand binding pocket. AcFaNaC is shown as a cartoon, with each subunit colored differently and side chains of key residues shown as sticks. The FMRFamide molecules are shown as sticks
Supplementary Video 3
Morph between apo and FMRFamide-bound AcFaNaC structures in a single subunit. AcFaNaC is shown as a cartoon, and the FMRFamide molecules are shown as sticks.
Supplementary Video 4
Morph between apo and FMRFamide-bound trimeric AcFaNaC structures. AcFaNaC is shown as a cartoon, and the FMRFamide molecules are shown as sticks.
Supplementary Data
Statistical source data.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data and unprocessed western blot.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, F., Dang, Y., Li, L. et al. Structure and mechanism of a neuropeptide-activated channel in the ENaC/DEG superfamily. Nat Chem Biol 19, 1276–1285 (2023). https://doi.org/10.1038/s41589-023-01401-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-023-01401-7
This article is cited by
-
Structural basis for excitatory neuropeptide signaling
Nature Structural & Molecular Biology (2024)