Abstract
Although nicotinamide adenine dinucleotide phosphate (NADPH) is produced and consumed in both the cytosol and mitochondria, the relationship between NADPH fluxes in each compartment has been difficult to assess due to technological limitations. Here we introduce an approach to resolve cytosolic and mitochondrial NADPH fluxes that relies on tracing deuterium from glucose to metabolites of proline biosynthesis localized to either the cytosol or mitochondria. We introduced NADPH challenges in either the cytosol or mitochondria of cells by using isocitrate dehydrogenase mutations, administering chemotherapeutics or with genetically encoded NADPH oxidase. We found that cytosolic challenges influenced NADPH fluxes in the cytosol but not NADPH fluxes in mitochondria, and vice versa. This work highlights the value of using proline labeling as a reporter system to study compartmentalized metabolism and reveals that NADPH homeostasis in the cytosolic and mitochondrial locations of a cell are independently regulated, with no evidence for NADPH shuttle activity.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Source data are provided for figures, extended data figures and supplementary figures. All supplementary figures, tables and notes are included in the Supplementary Information. The LC-MS files have been deposited in the MassIVE database under the accession code MSV000090926 (ftp://massive.ucsd.edu/MSV000090926/).
References
Lehninger, A., Nelson, D. & Cox, M. (eds) Lehninger Principles of Biochemistry 5th edn (W. H. Freeman and Company, 2008).
Katsyuba, E., Romani, M., Hofer, D. & Auwerx, J. NAD+. homeostasis in health and disease. Nat. Metab. 2, 9–31 (2020).
Di Lisa, F. & Ziegler, M. Pathophysiological relevance of mitochondria in NAD+. metabolism. FEBS Lett 492, 4–8 (2001).
Shestov, A. A. et al. Quantitative determinants of aerobic glycolysis identify flux through the enzyme GAPDH as a limiting step. eLife 3, e03342 (2014).
Wang, Y. et al. Saturation of the mitochondrial NADH shuttles drives aerobic glycolysis in proliferating cells. Mol. Cell 82, 3270–3283.e3279 (2022).
Borst, P. The malate-aspartate shuttle (Borst cycle): how it started and developed into a major metabolic pathway. IUBMB Life 72, 2241–2259 (2020).
Xiao, W., Wang, R. S., Handy, D. E. & Loscalzo, J. NAD(H) and NADP(H) redox couples and cellular energy metabolism. Antioxid. Redox Signal 28, 251–272 (2018).
Houtkooper, R. H., Cantó, C., Wanders, R. J. & Auwerx, J. The secret life of NAD+: an old metabolite controlling new metabolic signaling pathways. Endocr. Rev. 31, 194–223 (2010).
Blacker, T. S. & Duchen, M. R. Investigating mitochondrial redox state using NADH and NADPH autofluorescence. Free Radic. Biol. Med. 100, 53–65 (2016).
Lemons, J. M. et al. Quiescent fibroblasts exhibit high metabolic activity. PLoS Biol. 8, e1000514 (2010).
Wallace, D. C. Mitochondria and cancer. Nat. Rev. Cancer 12, 685–698 (2012).
Ducker, G. S. & Rabinowitz, J. D. One-carbon metabolism in health and disease. Cell Metab. 25, 27–42 (2017).
Lewis, C. A. et al. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol. Cell 55, 253–263 (2014).
MacDonald, M. J. Feasibility of a mitochondrial pyruvate malate shuttle in pancreatic islets. Further implication of cytosolic NADPH in insulin secretion. J. Biol. Chem. 270, 20051–20058 (1995).
Tanner, J. J., Fendt, S. M. & Becker, D. F. The proline cycle as a potential cancer therapy target. Biochemistry 57, 3433–3444 (2018).
Veech, R. L. et al. The ‘great’ controlling nucleotide coenzymes. IUBMB Life 71, 565–579 (2019).
Fan, J. et al. Quantitative flux analysis reveals folate-dependent NADPH production. Nature 510, 298–302 (2014).
Ye, J. et al. Serine catabolism regulates mitochondrial redox control during hypoxia. Cancer Discov 4, 1406–1417 (2014).
Martínez-Reyes, I. & Chandel, N. S. Mitochondrial one-carbon metabolism maintains redox balance during hypoxia. Cancer Discov 4, 1371–1373 (2014).
Phang, J. M. Proline metabolism in cell regulation and cancer biology: recent advances and hypotheses. Antioxid. Redox Signal 30, 635–649 (2019).
De Ingeniis, J. et al. Functional specialization in proline biosynthesis of melanoma. PLoS One 7, e45190 (2012).
Dang, L. & Su, S. M. Isocitrate dehydrogenase mutation and (R)-2-hydroxyglutarate: from basic discovery to therapeutics development. Annu. Rev. Biochem. 86, 305–331 (2017).
Dang, L. et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 462, 739–744 (2009).
Ward, P. S. et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 17, 225–234 (2010).
Gelman, S. J. et al. Consumption of NADPH for 2-HG synthesis increases pentose phosphate pathway flux and sensitizes cells to oxidative stress. Cell Rep. 22, 512–522 (2018).
Badur, M. G. et al. Oncogenic R132 IDH1 mutations limit NADPH for de novo lipogenesis through (D)2-hydroxyglutarate production in fibrosarcoma cells. Cell Rep. 25, 1680 (2018).
Liu, L. et al. Malic enzyme tracers reveal hypoxia-induced switch in adipocyte NADPH pathway usage. Nat. Chem. Biol. 12, 345–352 (2016).
Lee, W. N. et al. Mass isotopomer study of the nonoxidative pathways of the pentose cycle with [1,2-13C2]glucose. Am. J. Physiol. 274, E843–E851 (1998).
Ducker, G. S. et al. Reversal of cytosolic one-carbon flux compensates for loss of the mitochondrial folate pathway. Cell Metab. 23, 1140–1153 (2016).
Hvinden, I. C., Cadoux-Hudson, T., Schofield, C. J. & McCullagh, J. S. O. Metabolic adaptations in cancers expressing isocitrate dehydrogenase mutations. Cell Rep. Med. 2, 100469 (2021).
Cracan, V., Titov, D. V., Shen, H., Grabarek, Z. & Mootha, V. K. A genetically encoded tool for manipulation of NADP+/NADPH in living cells. Nat. Chem. Biol. 13, 1088–1095 (2017).
Kleih, M. et al. Direct impact of cisplatin on mitochondria induces ROS production that dictates cell fate of ovarian cancer cells. Cell Death Dis. 10, 851 (2019).
Marullo, R. et al. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS One 8, e81162 (2013).
Asensio-López, M. C. et al. Early oxidative damage induced by doxorubicin: source of production, protection by GKT137831 and effect on Ca2.ÿtransporters in HL-1 cardiomyocytes. Arch. Biochem. Biophys. 594, 26–36 (2016).
Gilliam, L. A. et al. Doxorubicin acts via mitochondrial ROS to stimulate catabolism in C2C12 myotubes. Am. J. Physiol. Cell Physiol. 302, C195–C202 (2012).
Trnka, J., Blaikie, F. H., Smith, R. A. & Murphy, M. P. A mitochondria-targeted nitroxide is reduced to its hydroxylamine by ubiquinol in mitochondria. Free Radic. Biol. Med. 44, 1406–1419 (2008).
Pollak, N., Dölle, C. & Ziegler, M. The power to reduce: pyridine nucleotides–small molecules with a multitude of functions. Biochem. J. 402, 205–218 (2007).
Maddocks, O. D., Labuschagne, C. F. & Vousden, K. H. Localization of NADPH production: a wheel within a wheel. Mol. Cell 55, 158–160 (2014).
Zhu, J. et al. Mitochondrial NADP(H) generation is essential for proline biosynthesis. Science 372, 968–972 (2021).
Tran, D. H. et al. Mitochondrial NADP(+) is essential for proline biosynthesis during cell growth. Nat. Metab. 3, 571–585 (2021).
Lunt, S. Y. & Vander Heiden, M. G. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 27, 441–464 (2011).
Balsa, E. et al. Defective NADPH production in mitochondrial disease complex I causes inflammation and cell death. Nat. Commun. 11, 2714 (2020).
Bradshaw, P. C. Cytoplasmic and mitochondrial NADPH-coupled redox systems in the regulation of aging. Nutrients 11, 504 (2019).
Yuan, J., Bennett, B. D. & Rabinowitz, J. D. Kinetic flux profiling for quantitation of cellular metabolic fluxes. Nat. Protoc. 3, 1328–1340 (2008).
Chen, L. et al. NADPH production by the oxidative pentose-phosphate pathway supports folate metabolism. Nat. Metab. 1, 404–415 (2019).
Intlekofer, A. M. et al. L-2-Hydroxyglutarate production arises from noncanonical enzyme function at acidic pH. Nat. Chem. Biol. 13, 494–500 (2017).
Teng, X., Emmett, M. J., Lazar, M. A., Goldberg, E. & Rabinowitz, J. D. Lactate dehydrogenase C produces S-2-hydroxyglutarate in mouse testis. ACS Chem. Biol. 11, 2420–2427 (2016).
Jang, C., Chen, L. & Rabinowitz, J. D. Metabolomics and isotope tracing. Cell 173, 822–837 (2018).
Yang, L. et al. Serine catabolism feeds NADH when respiration is impaired. Cell Metab. 31, 809–821.e806 (2020).
Spalding, J. L., Naser, F. J., Mahieu, N. G., Johnson, S. L. & Patti, G. J. Trace phosphate improves ZIC-pHILIC peak shape, sensitivity, and coverage for untargeted metabolomics. J. Proteome Res. 17, 3537–3546 (2018).
Yao, C. H. et al. Mitochondrial fusion supports increased oxidative phosphorylation during cell proliferation. eLife 8, pii e41351 (2019).
Chen, Y. J. et al. Lactate metabolism is associated with mammalian mitochondria. Nat. Chem. Biol. 12, 937–943 (2016).
Yao, C. H. et al. Dose-response metabolomics to understand biochemical mechanisms and off-target drug effects with the TOXcms software. Anal. Chem. 92, 1856–1864 (2019).
Acknowledgements
This work was supported by funding from National Institutes of Health grant nos. R35ES028365 (G.J.P.) and R24OD024624 (G.J.P.).
Author information
Authors and Affiliations
Contributions
X.N. and G.J.P. designed the study. X.N. prepared the samples and collected most of the experimental data. E.S. built the metabolic flux models and performed the computational analysis. S.J.G. contributed to experiments assessing relative PPP flux in IDH1 and IDH2 cells. L.W. conducted dose–response analysis by using the TOXcms R package. J.L.R. helped prepare HCT116 cells expressing TPNOX. X.N., E.S., L.P.S. and G.J.P. wrote the paper. M.S.-H. and all authors contributed to data analysis, data interpretation and approved the paper.
Corresponding author
Ethics declarations
Competing interests
G.J.P. is a scientific advisory board member for Cambridge Isotope Laboratories and founder of Panome Bio. The Patti Laboratory has a research collaboration agreement with Thermo Fisher Scientific.
Peer review
Peer review information
Nature Chemical Biology thanks Li Chen, Seth Parker and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Model of cytosolic and mitochondrial NADPH fluxes.
a, Model to assess the distribution of cytosolic NADPH fluxes in cells labeled with 3-2H glucose. b, Model to assess the distribution of mitochondrial NADPH fluxes in cells labeled with 4-2H glucose. PPP, pentose phosphate pathway; G6P, glucose 6-phosphate; P5C, pyrroline 5-carboxylic acid; NADPH[c], cytosolic NADPH; NADPH[m], mitochondrial NADPH; NNT, nicotinamide nucleotide transhydrogenase; PYCR, P5C reductase; PYCR1/2, subtypes of PYCR in mitochondria; PYCRL, subtype of PYCR in cytosol; MDH, malate dehydrogenase; ME, malic enzyme.
Extended Data Fig. 2 Effect of IDH1 and IDH2 mutations on proline, G6P, P5C, and malate labeling.
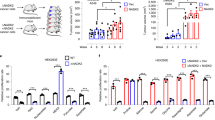
a, Time course of proline M + 2H enrichment in HCT116 wild-type cells labeled with 3-2H glucose. b, Time course of proline M + 2H enrichment in HCT116 wild-type cells labeled with 4-2H glucose. c, Proline M + 2H enrichment in cells labeled with 3-2H glucose for 48 hours. d, G6P M + 2H enrichment in cells labeled with 3-2H glucose for 48 hours. e, P5C M + 2H enrichment in cells labeled with 4-2H glucose for 48 hours. f, Malate M + 2H enrichment in cells labeled with 4-2H glucose for 48 hours. (c–f) Although differences in the ratio of proline to G6P labeling and the ratio of P5C to malate labeling are driven by the numerator in our cells, we include G6P and malate in our calculations to normalize potential differences in upstream metabolic processes that are independent of NADPH (for example, a change in glucose consumption). WT, wild-type HCT116 cells; mIDH1, mutant IDH1 cells; mIDH2, mutant IDH2 cells; G6P, glucose 6-phosphate; P5C, pyrroline 5-carboxylic acid; 2HG, 2-hydroxyglutarate. Values are mean ± s.d; n = 3 biologically independent samples (a–f). Statistically significant differences were calculated by using a one-way ANOVA followed by Dunnett’s multiple comparison test (c-f). NS= no significant difference.
Extended Data Fig. 3 Mutant IDH protein inactivation by inhibitors alters NADPH fluxes induced by mutant IDH.
To confirm that the observed changes in cytosolic and mitochondrial NADPH metabolism were caused by compartmentalized (D) 2-hydroxyglutarate (2HG) production, we repeated the analysis in the presence of pharmacological inhibitors that selectively inactivate mutant IDH protein. We used AGI-5198 to inhibit 2HG production by mutant IDH1 and enasidenib to inhibit 2HG production by mutant IDH2. As anticipated, AGI-5198 reduced intracellular 2HG levels in IDH1 mutants but did not influence intracellular 2HG levels in IDH2 mutants (a). Enasidenib had the opposite effect, reducing intracellular 2HG levels in IDH2 mutants but not IDH1 mutants (a). We treated cells with either AGI-5198 or enasidenib for 48 hours, while they were being labeled with 2H glucose. Neither inhibitor influenced the distribution of cytosolic or mitochondrial NADPH fluxes in wild-type cells. AGI-5198, but not enasidenib, did lead to a statistically significant change in the distribution of cytosolic NADPH fluxes in IDH1 mutants (b-d). Only enasidenib, on the other hand, influenced the distribution of mitochondrial NADPH fluxes in IDH2 mutants (e-h). These rescue experiments provide additional evidence that localized production of 2HG has compartment-specific effects on NADPH metabolism. a, Relative levels of intracellular 2HG from HCT116 wild-type cells, IDH1 mutants, and IDH2 mutants treated with AGI-5198 or Enasidenib. b, Ratio of proline to G6P enrichment in HCT116 wild-type cells labeled with 3-2H glucose is not affected by inhibitors of mutant IDH. c, Ratio of proline to G6P enrichment in IDH1 mutants labeled with 3-2H glucose. Inhibition of mutant IDH1 with AGI-5198 alters the distribution of cytosolic NADPH fluxes, but enasidenib does not. d, Ratio of proline to G6P enrichment in IDH2 mutants labeled with 3-2H glucose is not affected by inhibitors of mutant IDH. e, Ratio of P5C to malate enrichment in HCT116 wild-type cells labeled with 4-2H glucose is not affected by inhibitors of mutant IDH. f, Ratio of P5C to malate enrichment in IDH1 mutants labeled with 4-2H glucose is not affected by inhibitors of mutant IDH. g, Ratio of P5C to malate enrichment in IDH2 mutants labeled with 4-2H glucose. Inhibition of mutant IDH2 with enasidenib alters the distribution of mitochondrial NADPH fluxes, but AGI-5198 does not . WT, wild-type HCT116 cells; mIDH1, mutant IDH1 cells; mIDH2, mutant IDH2 cells. A concentration of 0.2 μM AGI-5198 and 0.1 μM enasidenib was used. Values are mean ± s.d; n = 3 biologically independent samples (a-g). Statistically significant differences were calculated by using a one-way ANOVA followed by Dunnett’s multiple comparison test (a-g).NS= no significant difference.
Extended Data Fig. 4 IDH1 and IDH2 mutations in GL261 and TF1 cells lead to compartment-specific alterations in NADPH metabolism.
We applied the same strategy outlined for HCT116 cells to assess changes in the distribution of cytosolic and mitochondrial NADPH fluxes in GL261 murine glioma cells harboring gain-of-function mutations in IDH1 and TF-1 erythroleukemia cells harboring gain-of-function mutations in IDH2. Consistent with our results from HCT116 cells, IDH1 mutations caused alterations in the distribution of cytosolic NADPH fluxes but did not lead to a statistically significant change in the distribution of mitochondrial NADPH fluxes (a-b). IDH2 mutations had the opposite effect of producing statistically significant changes in the distribution of mitochondrial NADPH fluxes but not cytosolic NADPH fluxes (c-d). a, Ratio of proline to G6P enrichment in GL261 cells labeled with 3-2H glucose. b, Ratio of P5C to malate enrichment in GL261 cells labeled with 4-2H glucose. c, Ratio of proline to G6P enrichment in TF-1 cells labeled with 3-2H glucose. d, Ratio of P5C to malate enrichment in TF-1 cells labeled with 4-2H glucose. Values are mean ± s.d; n = 3 biologically independent samples (a-d). Statistically significant differences were calculated by using a two-tailed t-test (a-d). NS= no significant difference.
Extended Data Fig. 5 Evaluating changes in PPP and one-carbon metabolism.
a, Relative lactate enrichment in cells labeled with 1,2-13C2 glucose for 12 hours. b, Relative glucose uptake as measured by the depletion of glucose from media after culturing cells for 12 hours. c, Enrichment of AMP in cells labeled with 2,3,3-2H3 serine for 48 hours. Values are mean ± s.d; n = 6 biologically independent samples (WT in a and b, c) and n = 3 biologically independent samples (mIDH1 and mIDH2 in a and b). WT, HCT116 wild-type cells; mIDH1, mutant IDH1 cells; mIDH2, mutant IDH2 cells; AMP, adenosine monophosphate. Statistically significant differences were calculated by using a one-way ANOVA followed by Dunnett’s multiple comparison test (a-b). NS= no significant difference.
Extended Data Fig. 6 Characterizing the effects of cisplatin and doxorubicin.
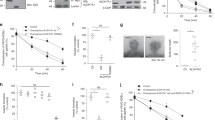
a, Dose-response analysis for cisplatin administered to HCT116 wild-type cells. Cell proliferation was assessed at increasing concentrations of drug. b, Dose-response analysis for doxorubicin administered to HCT116 wild-type cells. Cell proliferation was assessed at increasing concentrations of drug. c, Percent decreased viability of cells treated with 10 μM cisplatin for 48 hours. Data were obtained by using an MTT assay. Values are mean ± s.d; n = 8 biologically independent samples (a-c, n = 7 biologically independent samples for WT in c). Statistically significant differences were calculated by using a one-way ANOVA followed by Dunnett’s multiple comparison test (c). NS= no significant difference.
Supplementary information
Supplementary Information
Supplementary Notes 1–4, Figs. 1–4 and original TIF images of Supplementary Fig. 3a–c.
Supplementary Tables
The peak areas and calculated labeling percentages for proline, malate, P5C and G6P and the raw enrichment of lactate.
Supplementary Data 1
Source data for Supplementary Fig. 1.
Supplementary Data 2
Source data for Supplementary Fig. 2.
Supplementary Data 4
Source data for Supplementary Fig. 4.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Niu, X., Stancliffe, E., Gelman, S.J. et al. Cytosolic and mitochondrial NADPH fluxes are independently regulated. Nat Chem Biol 19, 837–845 (2023). https://doi.org/10.1038/s41589-023-01283-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-023-01283-9
This article is cited by
-
A genetically encoded tool to increase cellular NADH/NAD+ ratio in living cells
Nature Chemical Biology (2024)
-
Reporting NADPH fluxes
Nature Chemical Biology (2023)