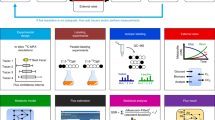
Abstract
Anaerobic microbial metabolism drives critical functions within global ecosystems, host–microbiota interactions, and industrial applications, yet remains ill-defined. Here we advance a versatile approach to elaborate cellular metabolism in obligate anaerobes using the pathogen Clostridioides difficile, an amino acid and carbohydrate-fermenting Clostridia. High-resolution magic angle spinning nuclear magnetic resonance (NMR) spectroscopy of C. difficile, grown with fermentable 13C substrates, informed dynamic flux balance analysis (dFBA) of the pathogen’s genome-scale metabolism. Analyses identified dynamic recruitment of oxidative and supporting reductive pathways, with integration of high-flux amino acid and glycolytic metabolism at alanine’s biosynthesis to support efficient energy generation, nitrogen handling and biomass generation. Model predictions informed an approach leveraging the sensitivity of 13C NMR spectroscopy to simultaneously track cellular carbon and nitrogen flow from [U-13C]glucose and [15N]leucine, confirming the formation of [13C,15N]alanine. Findings identify metabolic strategies used by C. difficile to support its rapid colonization and expansion in gut ecosystems.

Similar content being viewed by others
Main
Obligate anaerobes comprise the majority of species in the mammalian gut microbiota and include pathogens such as Clostridioides difficile. Anaerobic bacteria also modulate nutrient flow across global ecosystems1 and perform industrial fermentations of economic importance2. However, the metabolic pathways and nutrient requirements of anaerobes often differ substantively from those of model, aerotolerant species such as Escherichia coli or Bacillus subtilis, leaving many aspects of their metabolism poorly defined. This poor characterization limits efforts to harness anaerobe metabolism in applications of clinical, industrial or environmental importance.
C. difficile, the leading cause of hospital-acquired infections, colonizes gut environments through its fermentation of diverse carbon sources3, including carbohydrates and amino acids4,5,6. The pathogen releases toxins to obtain nutrients from damaged mucosa as its growth exceeds the carrying capacity of gut environments7. Defining how C. difficile recruits cooccurring fermentation pathways with systems supporting energy generation and growth has been challenging8,9. However, means to definitively track the use of growth-promoting fermentable substrates, and associated recruitment of metabolic pathways, offers opportunities to prevent and treat infections with approaches that need not rely solely on antibiotics.
High-resolution magic angle spinning (HRMAS) nuclear magnetic resonance (NMR) spectroscopy supports studies of real-time metabolism in living cells10,11,12, and is particularly suited to the study of anaerobes as the sealed rotor chamber can maintain an anaerobic environment12. HRMAS NMR rotates samples at a ‘magic angle’ of 54.74° relative to the magnetic field during NMR spectrum measurement, improving the sensitivity of signal detection in colloidal or semisolid samples13. Detailed studies of metabolism can thus be achieved with a low input biomass of cells12. When coupled with cellular metabolism of uniformly carbon-13 (13C) labeled substrates, HRMAS NMR’s sensitivity enables definitive tracking of carbon flow through complex metabolic pathways.
Metabolic modeling systems link experimentally obtained substrate and metabolite fluxes to cellular pathways and genes14,15,16,17. Among modeling approaches, dynamic flux balance analysis (dFBA) simulates time-dependent recruitment of metabolic pathways on an organismal scale given a set of reaction flux constraints and a biological objective such as biomass or adenosine triphosphate (ATP) production18. Existing dFBA approaches estimate exchange fluxes from static measurements of the media composition over time18,19 for which quantitative platforms, including gas chromatography and mass spectrometry (GC–MS) analyses of C. difficile-produced metabolites, have been used20. However, the use of NMR to constrain dFBA simulations has been limited by means to translate NMR signals into credible estimated concentrations due to issues in NMR spectral resolution, signal-to-noise levels and susceptibility of 13C NMR peaks to amplitude distortions from the nuclear Overhauser effect (NOE)21. With means to overcome these limitations, HRMAS NMR offers a promising approach to support dFBA given its nondestructive measurement of isotopic flux in minute quantities of living cells12,22.
We leveraged HRMAS 13C NMR to constrain dFBA within genome-scale metabolic models to define complex dynamics in C. difficile’s metabolism. HRMAS NMR spectra were acquired from living C. difficile cells fermenting defined 13C-labeled substrates over time (Fig. 1a). The 13C NMR integrated signal curves were then normalized using experimental standards to provide constraints for dFBA simulations (Methods, Estimation of exchange fluxes for dFBA and Fig. 1b). Analyses revealed alanine production as a central metabolic integration point in amino acid-fermenting Clostridia to support cooccurring oxidative and reductive reactions across amino acid and glycolytic metabolism. The confirmation of model predictions leveraged a generalizable NMR approach to simultaneously track carbon and nitrogen flow through cellular metabolism, using amplification of signal from weak NMR-active nuclei, such as nitrogen-15 (15N), through strong NMR-active nuclei, such as 13C (Fig. 1c).
a, HRMAS NMR resolves live-cell anaerobic metabolism of 13C-labeled substrates. A defined medium containing a 13C-labeled substrate is inoculated with C. difficile cells in an HRMAS rotor insert. Successive 1H- and 13C NMR spectra of the growing cells are acquired throughout log-phase growth to monitor metabolism of the labeled substrate. NMR spectra are processed, and peaks are assigned to metabolites using 1H-13C HSQC spectra and reference data. b, NMR signal trajectories inform dFBA simulations to identify metabolic integration points. Logistic curves for metabolites are fit to integrated 13C-signal trajectories and scaled to estimated concentrations using information from standard solutions measured by GC or NMR. Estimated metabolite exchange fluxes are derived from the logistic curves representing multiple NMR runs to constrain dFBA simulations. Metabolic integration points are identified where dFBA solutions predict substrates to exchange electrons or functional groups. c, 13C NMR of 13C- and 15N-labeled substrates confirms dFBA predictions of nutrient flow. C. difficile cells are grown in defined media containing 13C- and 15N-labeled substrates under NMR acquisition. 15N flow to 13C backbones is measured by quantifying the relative areas of split 13C NMR subpeaks at the 13C-alanine alpha carbon.
Results
HRMAS 13C NMR elucidates C. difficile’s complex metabolism
To investigate the progression of co-occurring amino acid and glycolytic fermentations in C. difficile, we measured HRMAS NMR time series of proton (1H) and 13C NMR spectra from living C. difficile cells. Cultures were grown in modified minimal medium (MMM) that replaced a given carbon source at natural isotope abundance with its uniformly labeled carbon-13 isotopologue: l-[U-13C]proline, l-[U-13C]leucine or [U-13C]glucose (Fig. 1a). C. difficile cultures grown under the conditions of HRMAS acquisition demonstrated consistent metabolic findings with conventional anaerobic cultures (Extended Data Fig. 1, Supplementary Table 1 and Methods, Nonspinning control experiments).
As a Stickland amino acid-fermenting Clostridium6, C. difficile preferentially metabolizes amino acids, such as proline and leucine, with simple sugars, such as glucose23. These substrates are known to drive rapid pathogen growth in vivo9, a prerequisite for the development of symptomatic disease23,24. C. difficile’s proline reductase reduces proline to a single metabolite, 5-aminovalerate4,25. Biochemical and protein interaction studies have identified coupling of proline reductase activity to proton ejection by the bacterial Rnf complex, which supports ATP synthesis26,27. HRMAS NMR of C. difficile cultures growing in media containing l-[U-13C]proline revealed complete proline consumption within 5 hours (h), producing [U-13C]5-aminovalerate (Fig. 2a,b, Extended Data Fig. 2 and Supplementary Fig. 1).
a, HRMAS NMR stack plot of growth in MMM with 7.0 mM [U-13C]proline. The legend shows color-coding of input proline (dark blue) and 5-aminovalerate (dark red). The x axis shows 13C NMR chemical shift (ppm), the y axis shows time (h) and z axis shows NMR signal (unitless). b, Logistic plots depicting estimated concentration (mM) of [U-13C]proline and [U-13C]5-aminovalerate versus time (h). Trajectories are calculated and normalized as described in the Methods (section Aligning the time axes and calculating average logistic coefficients). Bold lines depict mean trajectories across three experimental replicates; shaded regions depict the 95% confidence interval of the mean. c, HRMAS NMR stack plot of growth in MMM with 7.6 mM [U-13C]leucine. The legend shows carbons in leucine (blue-green) and detected metabolites; axes as in a. The 25 ppm peaks (gray) of isovalerate and isocaproate could not be resolved due to extensive overlap. d, Logistic plots depicting estimated concentration of [U-13C]leucine and detected metabolites versus time. Axes and curves as in b. e, HRMAS NMR stack plot of growth in MMM with 27.5 mM [U-13C]glucose. The legend shows color-coding of carbons in glucose (blue) and metabolites appearing over 36 h of cellular metabolism; axes as in a. f, Logistic plots depicting estimated concentration of [U-13C]glucose and detected metabolites versus time. Axes and curves as in b. g,h, 13C NMR time series of C. difficile cultures in MMM with 30 mM [U-13C]proline with (g) or without (h) 100 µM sodium selenite. Legend shows color-coding of input proline (dark blue) and 5-aminovalerate (dark red); axes as in a.
In contrast, C. difficile ferments l-leucine through separate oxidative and reductive pathways5,24. Oxidative fermentation of leucine to isovalerate and CO2 produces two reducing equivalents of ferredoxin and one equivalent of ATP, whereas the reductive leucine pathway yields isocaproate and consumes four net reducing equivalents5,24,28. HRMAS NMR revealed complete metabolism of l-[U-13C]leucine within 13 h, with levels of [13C]isovalerate and [13C]isocaproate rising over 2.7–8.7 and 7.4–13.0 h, respectively (Fig. 2c,d, Extended Data Fig. 3 and Supplementary Fig. 2).
C. difficile ferments glucose through progressive oxidative and reductive pathways that support the organism’s energetic and metabolic needs. The highest flux oxidative pathways convert glycolytic pyruvate to acetate, with production of ATP and further extraction of electrons that can feed into the Rnf system. Corresponding high-flux reductive pathways produce ethanol, lactate, butyrate and butanol with regeneration of electron carriers, as well as reactions, including electron bifurcating systems, that extract further energy to support energetic needs6,28. HRMAS NMR of C. difficile grown with [U-13C]glucose identified [13C]acetate at 7 h and [13C]alanine at 10 h (Fig. 2e,f). Metabolic products of reductive glucose metabolism were detected by 13 h with production of [13C]ethanol, followed by [13C]lactate at 21 h, [13C]butyrate at 24 h and n-[13C]butanol at 35 h (Fig. 2e,f, Extended Data Fig. 4 and Supplementary Fig. 3).
To assess the responsiveness of live-cell HRMAS NMR to metabolic perturbations, we next compared C. difficile’s proline reductase metabolism, a selenium-dependent pathway25,29, in the presence of an excess of 30 mM [U-13C]proline. Compared to C. difficile grown in MMM with 100 µM sodium selenite, cells grown in selenium-deficient MMM produced only 7% as much [U-13C]5-aminovalerate signal (compare Fig. 2g with h).
Using NMR data to constrain metabolic simulations
We next estimated metabolite exchange fluxes from the HRMAS 13C NMR trajectories, linking NMR datasets to the supporting metabolic pathways, genes and their dynamic recruitment over the experimental time series (Fig. 1b). To estimate metabolite concentration trajectories, the integrated NMR signal over time for each 13C compound was first fit to a logistic curve, using the equation:
where L is the upper asymptote, k is the growth rate and x0 is the inflection point of the sigmoidal curve (Supplementary Table 2). For the U-13C substrates, the constant C accounts for remaining substrate after 36 h; for the products, C equals zero. Final concentrations of 13C-labeled products were estimated using the known U-13C substrate concentration, ratios of 13C NMR peak area and empirically determined correction factors for NOE-induced signal amplification (Estimation of exchange fluxes for dFBA). Logistic equation (1) was scaled using estimated concentrations across replicate runs, and differentiated to yield exchange flux constraints for dFBA simulations (Fig. 2b,d,f and Supplementary Table 3):
dFBA predicts rapid ATP generation by Stickland metabolism
dFBA simulations used icdf843, an updated metabolic model for C. difficile (Metabolic modeling and Supplementary Table 4). Model exchange fluxes for NMR-detected metabolites were constrained to the 95% confidence bounds of the logistic derivative equation (2) evaluated over a simulated 36-h time series (Metabolic modeling and Supplementary Table 5). Solutions used ATP hydrolysis as the biological objective to provide consistency across phases of growth and sporulation (Supplementary Table 6). Flux variability analysis (FVA) solutions, computed at each simulated timepoint, estimated flux bounds for reactions accommodating an objective flux within 0.5% of the optimal value (Supplementary Tables 7 and 8).
dFBA simulations predicted three stages of metabolism with progressive recruitment of oxidative reactions driving ATP synthesis (Fig. 3a) and co-occurring reductive reactions to accept electrons and sustain oxidative flux. Model simulations predicted 36.1% of overall ATP synthesis during the first 10 h of metabolism with peak production occurring at 6.2 h. Metabolism during this period was marked by the rapid oxidative fermentation of leucine (Figs. 3a,b and 4, reaction 1) with progressive recruitment of oxidative Stickland amino acid fermentation pathways for isoleucine (Fig. 3a,b, reaction 2), valine (Fig. 3a,b, reaction 3) and cysteine (Fig. 3b, reaction 9). The substrate-level phosphorylation reactions from oxidative amino acid metabolism were estimated to comprise 27.6% of ATP production during this interval. Simulations predicted an additional 38.9% of ATP production as oxidative glycolytic (Figs. 3a,c and 4, reactions 5 and 6) and mixed-acid fermentation reactions were recruited (Figs. 3a,c and 4, reaction 7). dFBA solutions predicted progressive recruitment of reductive pathways during this period supporting the combined oxidative fermentations, starting with the evolution of molecular hydrogen from C. difficile’s hydrogenase system (Figs. 3d and 4, reaction 11), followed by Stickland reductive reactions for leucine and proline (Figs. 3e and 4, reactions 12 and 13).
a–i, dFBA-predicted reaction fluxes over 36 h of metabolism (x axis). The y axis shows inferred flux in mM h−1. a, Inferred flux of reactions contributing to the production of ATP versus time, including isovalerate kinase (light green, 1), 2-methylbutyrate kinase (yellow-green, 2), isobutyrate kinase (dark green, 3), ATP synthase (light gray, 4), PGK (dark blue, 5), pyruvate kinase (purple, 6), acetate kinase (red, 7) and butyrate kinase (yellow, 8). b–i, The model-inferred recruitment of oxidative and reductive pathways, including metabolic integration between amino acid and glycolytic metabolism. Bold lines depict FBA-predicted optimal metabolic fluxes; shaded regions depict FVA-predicted flux tolerances supporting objective flux within 0.5% of the optimal solution. b, Estimated flux of oxidative Stickland fermentations represented by cystathionase (purple, 9), isovalerate kinase (light green, 1), 2-methylbutyrate kinase (yellow-green, 2) and isobutyrate kinase (dark green, 3). c, Estimated glycolytic flux represented by PGK (dark blue, 5) and oxidative mixed-acid fermentations represented by pyruvate:ferredoxin oxidoreductase (PFOR; pink, 10) and acetate kinase (red, 7; *shaded FVA upper bound (1,000 mM h−1) is not displayed because it exceeds the axes limits). d, Reductive hydrogen production by iron hydrogenase (orange, 11). e, Reductive Stickland fermentation represented by isocaprenoyl-CoA reductase (light red, 12) and proline reductase (dark red, 13). f, Reductive mixed-acid fermentations represented by ethanol dehydrogenase (ADH; green, 14) and butyrate kinase (yellow, 8). g, Wood–Ljungdahl acetogenesis represented by acetyl-CoA synthase (blue-green, 15). h, Membrane-associated ATP synthesis represented by ATP synthase (light gray, 4) and the gradient-producing Rnf complex (medium gray, 16). i, Nitrogen cycling via alanine transaminase (medium blue, 17, *shaded FVA upper and lower bounds (± 1,000 mM h−1) are not displayed because they exceed the axes limits) and glutamate dehydrogenase (light blue, 18). Positive glutamate dehydrogenase flux depicts the forward reaction oxidizing glutamate to 2-oxoglutarate; negative flux, below the x axis, depicts the reverse reaction.
Diagram shows an updated metabolic map for C. difficile highlighting alanine’s role in nitrogen cycling (blue) and integration of glycolytic and mixed-acid fermentations (yellow) with Stickland oxidative (green) and reductive (dark red) reactions that release abundant amino nitrogen. The map also includes the Wood–Ljungdahl pathway (blue-green) and iron hydrogenase (‘Hyd’), predicted by dFBA to serve as key electron sinks and the Rnf-ATPase system responsible for ATP-producing electron transfer between oxidative and reductive fermentations (gray). Numbered reactions correspond to the reaction legend in Fig. 3 and Supplementary Table 10. The red-lined box indicates central reactions linking pyruvate, acetate and alanine across glycolytic and Stickland fermentations.
Simulations estimated 49.8% of overall ATP synthesis to occur over 10–23 h of metabolism, of which 84.1% was predicted to originate from glycolytic and mixed-acid fermentations, peaking at 14 h. After the depletion of leucine and proline pools for reductive metabolism, model simulations predicted recruitment of solvogenic ethanol fermentation (Figs. 3f and 4, reaction 14) and continued hydrogenase flux as the primary electron sinks.
Late-stage metabolism, occurring over 24–36 h, was predicted to account for the remaining 14.1% of overall ATP synthesis, marked by a shift to reductive, solvogenic reactions producing butyrate and n-butanol (Figs. 3a,f and 4, reaction 8), along with Wood–Ljungdahl acetogenesis (Figs. 3g and 4, reaction 15). Substrate-level phosphorylation in butyrate fermentation contributed an estimated 6.1% of ATP synthesis during this interval, whereas residual oxidative glucose fermentation was predicted to sustain 71.3% of ATP synthesis during this time.
Throughout all three stages of metabolism, dFBA simulations predicted that the Rnf complex (Figs. 3h and 4, reaction 14 and redox balance and energy generation) harnessed the energy cascade between oxidative and reductive metabolism to generate proton gradients and ATP production through C. difficile’s F-type ATP synthase (Figs. 3a,h and 4, reaction 7). Model solutions estimated a peak in membrane-associated ATP synthesis at 6.4 h, concurrent with the maximum reductive flux from amino acid fermentations. Thereafter, simulations predicted ATP generation to be sustained primarily via electron transfer from mixed-acid fermentation reactions to the Rnf complex and C. difficile’s F-type ATP synthase (Figs. 3h and 4, reaction 7).
dFBA predicts glycolysis-Stickland intergration at alanine
dFBA solutions, informed by HRMAS 13C NMR trajectories, predicted the transamination of pyruvate to alanine (Figs. 3i and 4, reaction 15) as a central integration point for glycolytic and Stickland amino acid metabolism to support energy generation, nitrogen handling and cellular growth (Fig. 4, red-lined box). Model solutions predicted net oxidative deamination of amino acids over the first 10 h of metabolism (Figs. 3i and 4, reaction 16), particularly from the rapid fermentation of leucine. By 11 h, with the progressive recruitment of high-flux glycolytic metabolism, solutions predicted the transamination of pyruvate to alanine, with leucine-origin ammonia flowing through glutamate to supply 56.4% of alanine’s amino nitrogen (Fig. 4, amino nitrogen flow and Supplementary Table 6).
Alternate dFBA objectives enhance model predictions
To evaluate the consistency of model predictions for timed metabolic progression and associated points of pathway integration, we performed dFBA and FVA simulations using alternate model objectives of biomass production and exopolysaccharide synthesis (Supplementary Fig. 4). Notably, the inferred pathway fluxes remained consistent across objectives with modest differences in flux magnitudes and timing for some reactions. In the case of cysteine fermentation (Supplementary Fig. 4b,k,t, reaction 9), cystathionase activity was predicted to supply central carbon pools during early metabolism under ATP hydrolysis. In constrast, maximum cystathionase flux occured during late metabolism when using biomass as the objective. The greatest difference occurred when using exopolysaccharide as the objective where flux occurred consistently throughout the time series, with a maximum during the first 10 h of metabolism, per shunting of produced pyruvate into gluconeogenesis reactions to generate glucose for expolysaccharide biosynthesis.
Additional nuances in inferred reaction fluxes among the model objectives included increased glycolytic flux in the biomass simulation and decreased glycolytic flux in the exopolysaccharide simulation versus the ATP hydrolysis condition, as represented by phosphoglycerate kinase (PGK) (Supplementary Fig. 4c,l,u, reaction 5). The negative PGK flux around 6 h, under the exopolysaccharide objective, inferred diversion of products via the reverse reaction into gluconeogenic pathways to supply the exopolysaccharide synthesis pathway with glucose residues.
Aside from the aforementioned differences, the inferred flux trajectories remained similar to the ATP hydrolysis objective. All three objectives predicted the timed progression from amino acid to glycolytic fermentations, and alanine’s biosynthesis as a metabolic integration point supporting the transfer of amino groups from deaminating Stickland reactions to glycolytic pyruvate.
Concurrent NMR tracking of 15N and 13C through metabolism
To confirm model predictions of amino nitrogen flow from fermented leucine to glycolytic pyruvate, we developed an NMR approach to simultaneously track cellular carbon and nitrogen flow. Tracking of less sensitive NMR-active nuclei, such as 15N, through cellular metabolism has been more challenging than that of more sensitive NMR-active nuclei such as 13C and 1H, as 15N produces 15-fold less signal than 13C30. However, NMR J-coupling between 13C and covalently bound 15N induces predictable patterns of nuclear spin–spin splitting and isotope-effect shifting in 13C signals31,32,33, enabling detection of the less sensitive 15N nucleus in the more sensitive 13C NMR spectrum (Analyses of 15N-13C peak splitting).
To confirm the feasibility of amplifying signal from less sensitive NMR nuclei in the spectrum of more sensitive NMR nuclei, we first evaluated 13C-induced splitting of acetate’s peaks in the 1H spectrum of cells grown with [U-13C]glucose. Signal from the methyl hydrogens of [13C2]acetate was split into double-doublet peaks, indicating well-resolved 1H spin coupling with the 13C nucleus in the methyl group (J of roughly 34 Hz) and long-range coupling with the 13C nucleus in the carboxyl group (J of roughly 53 Hz; Extended Data Fig. 6b). Moreover, the amplification of 13C signal in the 1H spectrum showed 30 times higher signal-to-noise ratio (S/N) than in the 13C spectrum alone (Extended Data Fig. 6b), demonstrating that spin–spin splitting in a more sensitive NMR spectrum (1H) offers enhanced detection of comparatively less sensitive NMR nuclei (13C).
Leveraging this property of NMR physics, we tracked simultaneous flow of 13C backbones from [U-13C]glucose and 15N amino groups from [15N]leucine to form [13C,15N]alanine (Figs. 1c and 4, nitrogen cycling).
NMR-detected 15N-13C bonding confirms metabolic integration
HRMAS 13C NMR time series of C. difficile grown with [U-13C]glucose and natural-abundance leucine revealed [2,3-13C]alanine and [U-13C]alanine in a 1:1 ratio (Fig. 5a,b, Extended Data Fig. 5a and Supplementary Table 9), indicating substantial assimilation of 12CO2 with [U-13C]acetate, an activity reported to occur via pyruvate:ferredoxin oxidoreductase in many species of Clostridia34. C. difficile grown in the presence of [U-13C]glucose and [15N]leucine showed 15N-induced splitting (J ≅ 5.6 Hz) and isotope-effect shifting (δ ≅ 0.025 parts per million (ppm)) of the 13C peaks associated with alanine’s alpha carbon and mixed populations of [15N]alanine and [14N]alanine (Fig. 5c,d, Extended Data Fig. 5b and Supplementary Table 9). Although [15N]leucine represented only 33% of amino-group nitrogen in the starting media, 57% (standard deviation (s.d.), 4%) of [13C]alanine carried the 15N isotope (Fig. 5e), confirming enriched transfer of the 15N amino group from fermented [15N]leucine to [13C]alanine.
a, 13C NMR spectra for alanine’s alpha carbon (53.0–53.8 ppm) after growth in MMM media with 27.5 mM [U-13C]glucose and 7.6 mM natural-abundance leucine for 36 h. Bold black line with gray shading depicts the mean 13C NMR signal ± s.e.m. across three biological replicates. Red-dotted lines indicate J-coupled peaks associated with [U-13C]alanine. Orange-dotted lines indicate J-coupled peaks associated with [2,3-13C]alanine resulting from 12CO2 assimilation with [U-13C]acetate, via pyruvate:ferredoxin oxidoreductase. b, Time series of J-coupled peaks at alanine’s alpha carbon over 24 h of metabolism, color coded as shown in b. c, 13C NMR spectra after growth in 27.5 mM [U-13C]glucose and 7.6 mM [15N]leucine showing J-coupled split peaks from [U-13C,15N] alanine (blue) or [2,3-13C,15N] alanine (green). Purple lines show peaks with a mixture of [U-13C,15N]alanine and [U-13C, 14N]alanine; yellow-green lines show peaks with [2,3-13C,15N]alanine and [2,3-13C, 14N]alanine. 13C NMR signal curve displays center and variability as in a. d, Time series of J-coupled peaks, color coded as shown in c. * The asterisk over the aqua-colored peak indicates combined [2,3-13C,14N]alanine, [2,3-13C,15N]alanine and [U-13C, 15N]alanine. e, Table shows percentages of 14N and 15N amino nitrogen in starting media with [U-13C]glucose and [15N]leucine, and resulting integrated 14N-13C and 15N-13C percentages (± s.d.) on alanine after 36 h of growth (Supplementary Table 9). N = 3 biological replicates to assess variability in the 15N:14N isotope ratio.
Discussion
We illustrate advancements in HRMAS NMR and genome-scale metabolic modeling to track single carbon and nitrogen flow through complex anaerobe metabolism. The sealed rotor chamber used in HRMAS NMR maintains an anaerobic environment, ensuring maintenance of reducing conditions, conservation of mass and biological containment. The nondestructive nature of HRMAS NMR spectroscopy enables high-resolution analyses of living cells in small reaction volumes, on the order of tens of microliters, whereas alternatives using GC, MS or regular solution NMR often require orders of magnitude more volume and cellular mass to support longitudinal analyses. The acquisition of HRMAS NMR datasets is also far more rapid and does not require further extraction or preparation. Additionally, we show that HRMAS NMR detects metabolic shifts accompanying defined perturbations in nutrient availability as demonstrated by the impact of selenium limitation on C. difficile’s metabolism of [U-13C]proline by its proline reductase selenoenzyme25.
Longitudinal tracking of fermentable 13C-labeled substrates supports high-resolution monitoring of individual pathways within complex nutrient conditions. The cellular scope of genome-scale metabolic models provides means to incorporate multiple NMR datasets tracking fermentable substrates into a unified dFBA solution with inference of flux through pathways not directly captured by NMR analyses and of cellular or experimental objectives that may include ATP generation, biomass or production of industrially and physiologically important metabolites. To overcome previous limitations in using NMR time series data for genome-scale predictions of cellular metabolism, we present approaches to transform integrated 13C NMR trajectories into flux estimates to support dFBA simulations (Estimation of exchange fluxes for dFBA). The approaches accommodate linear as well as complex branching pathways, such as for proline and glucose fermentation, respectively.
Our use of 15N-13C J-coupling to amplify less NMR-sensitive 15N nuclei with more sensitive 13C NMR presents a conceptual foundation for the simultaneous tracking of atomic species to determine their chemical and biological relevance. 13C NMR time series quantified enriched 15N flow from fermented [15N]leucine to glucose-origin 13C backbones, confirming alanine formation as a central integration point between glycolytic and Stickland fermentations. This use of NMR J-coupling may be extended to covalent bonding with other NMR-active nuclei such as 1H or 17O to track distinct spin-active nuclei in an NMR time series and experimentally confirm metabolic integration points predicted by dFBA (Analyses of 15N-13C peak splitting).
Our findings inform in vivo behaviors of C. difficile by illustrating how the pathogen rapidly recruits amino acid fermentation pathways when presented with abundant fermentable amino acids and carbohydrates, conditions that occur in vivo after antibiotic ablation of the microbiota9. NMR-informed, dFBA solutions indicated early recruitment of Stickland oxidative and reductive pathways to drive rapid ATP generation via substrate-level phosphorylation reactions and via C. difficile’s membrane-associated ATP synthase, whereas glycolytic and mixed-acid fermentation pathways were predicted to provide metabolic support as pools of fermentable amino acids were depleted. The consistency of dFBA solutions among differing model objectives demonstrates the use of this approach to thoroughly interrogate complex microbial metabolism and enhance the robustness of predictions, as well as to identify specific nuances in metabolic and cellular behaviors that may occur when optimizing for a particular objective.
NMR time series and genome-scale metabolic analyses also identified a unique strategy Stickland fermenters use to integrate Stickland metabolism with high-flux glycolytic metabolism, generating alanine to support cellular growth, energy generation, and more energy-efficient nitrogen handling20,35. dFBA simulations predicted a two-phase process for nitrogen flow, the first phase being the release of abundant ammonia from oxidative deamination of amino acids during Clostridial Stickland fermentation (Figs. 3b,e,i and 4, reaction 18, nitrogen cycling)25. The second phase is characterized by the reassimilation of released ammonia by glutamate dehydrogenase and concomitant transamination of pyruvate to alanine (Figs. 3i and 4, reactions 17 and 18). HRMAS 13C NMR of C. difficile cultures grown with [U-13C]glucose and l-[15N]leucine confirmed model predictions of enriched nitrogen flow from leucine to alanine formed with carbon backbones from glycolytic pyruvate (Figs. 4 and 5c,d, nitrogen cycling). After the depletion of preferred Stickland acceptors (Fig. 3e, reactions 12 and 13), alanine’s use as a nitrogen sink consumes reducing equivalents, regenerating oxidized electron carriers for ATP-producing oxidative reactions in high-flux glycolytic and mixed-acid fermentations (Figs. 3i and 4, reactions 17 and 18)35 and further supports cellular systems in protein and peptidoglycan synthesis36, energy storage28,37 and osmotic balance38,39.
Live-cell HRMAS 13C NMR with dynamic metabolic modeling provides a unified methodology to define cellular-scale anaerobic metabolism for diverse applications. This analytic approach can support further analyses of prokaryotic physiology including microbial responses to antibiotics or optimization of conditions to produce bioactive or industrially important chemicals from different input feedstocks.
Methods
Strains
A pathogenicity locus (PaLoc)-deleted strain of C. difficile ATCC 43255 was generated that lacked the tcdB, tcdE and tcdA genes to reduce biohazard risks for NMR analyses. The deletion mutant was created using a toxin-mediated allele exchange method40. Briefly, approximately 800 bp of DNA flanking the pathogenicity locus were amplified by PCR from C. difficile ATCC 43255 using the primers in Supplementary Table 11. Purified PCR products were cloned into the PmeI site of the pMSR0 vector using NEBuilder HiFi DNA Assembly. The resulting plasmid was transformed into E. coli strain NEB10β (New England Biolabs) and insert verified by sequencing. E. coli strains were cultured aerobically at 37 °C in Luria-Bertani (LB) medium or LB agar supplemented with chloramphenicol (15 μg ml−1). The plasmid was then transformed into E. coli HB101 (RP4) and conjugated into C. difficile ATCC 43255 that had been heat-shocked at 50 °C for 15 min. Transconjugants were selected on Brain Heart Infusion (BHI) agar plates with cycloserine (250 μg ml−1), cefoxitin (25 μg ml−1) and thiamphenicol (15 μg ml−1). Allelic exchange was performed as described40. This strain was shown to be nontoxigenic to human fibroblasts using the methods described in Girinathan et al.9.
Strain culture conditions
The ΔPaLoc strain of ATCC 43255 was cultured for 12 h in supplemented BHI media (Remel). Cells were spun and washed three times in prereduced PBS, prepared in molecular-clean water and diluted to introduce 100,000 cells into HRMAS NMR rotor inserts for analyses. Preparations were serially diluted and plated to Brucella agar (Remel) to quantitate vegetative cells and spores used in input preparations. Spore counts after 12 h of culture in supplemented BHI media were <0.1% of vegetative cells.
C. difficile MMM (pH 7.2) was prepared by supplementing C. difficile minimal medium (CDMM)41 with 100 µM sodium selenite and 0.5% glucose. MMM was prepared by substituting [U-13C]glucose (d-glucose-13C6, 99 atom), l-[U-13C]proline (l-proline-13C5, 99 atom %) or l-[U-13C]leucine (l-leucine-13C6, 98 atom %) in place of its natural carbon-isotope abundance analog at the same concentration (Sigma-Aldrich). Studies evaluating flow of amino nitrogen from Stickland-fermented leucine to [U-13C]pyruvate generated in glycolysis used l-[15N]leucine (Sigma-Aldrich).
HRMAS Kel-F rotor inserts (Bruker BioSpin Corporation) were loaded with approximately 100,000 CFU in an anerobic chamber to defined MMM. The insert was sealed and removed from the chamber for NMR analyses.
After analyses, rotor contents were checked for pH, serially diluted, plated to Brucella agar and incubated anaerobically at 37 °C for cellular biomass and absence of contaminating species. Contents were also Gram stained and visualized by optical microscopy to confirm cellular morphology. Cellular growth occurring in the rotors is shown in Supplementary Table 1. The pH remained ranged from 7.17 to 7.27 after 36 h of analyses.
HRMAS NMR
HRMAS NMR measurements were performed on a Bruker Avance III HD 600 MHz spectrometer (Bruker BioSpin Corporation). The sealed Kel-F insert with live cells loaded in the anaerobic chamber was placed with 2 µl of D2O (for field locking) in a 4 mm zirconia rotor before the rotor was sealed and introduced into the triple-resonance HRMAS probe. One- and two-dimensional (1D, 2D) 1H and 13C NMR were conducted at 37 °C with a spin-rate of 3,600 ± 2 Hz. 1D time series spectra were measured alternately and continuously for 1H NOESY (NOE spectroscopy) with water suppression (roughly 13 min) and for proton-decoupled 13C (roughly 43 min) throughout the length of the experimental time. 2D 1H COSY (correlated spectroscopy, roughly 3 h and 49 min), proton-decoupled 13C COSY (roughly 3 h and 30 min) and 13C-decoupled 1H-13C HSQC (heteronuclear signal quantum coherence, roughly 3 h and 38 min) spectra were inserted in the between the 1D time series. Magnetic resonance spectra were processed using the TopSpin 3.6.2 (Bruker BioSpin Corporation), as well as with NUTS (Acorn NMR Inc.).
Nonspinning control experiments
To evaluate the impact of spinning of the HRMAS NMR rotor on cellular morphology, metabolism and biomass, sealed HRMAS inserts were prepared as described in the section Strain culture conditions and incubated without spinning for the duration of runs that evaluated a paired insert subjected to HRMAS NMR. Single 1D and 1D 1H and 13C NMR spectra were acquired from the nonspinning insert after the end of the incubation period for comparison with the spun insert, as described in the section HRMAS NMR. Rotor contents were analyzed as described in the section Strain culture conditions (Supplementary Table 1). Analyses showed comparable metabolic profiles. By Gram stain, rotors maintained outside the NMR showed more advanced progression of cultures to a postsporulation state, as evidenced by the presence of spores and lysed mother cells (Extended Data Fig. 1).
Identification of metabolite productions with 2D NMR
13C-labeled metabolites produced from [U-13C]glucose, l-[U-13C]proline and l-[U-13C]leucine in live cells were identified through 2D NMR (Extended Data Fig. 6) according to reported 13C and 1H chemical shift values availed from Human Metabolome Database (HMDB, https://hmdb.ca) and Biological Magnetic Resonance Bank (BMRB, https://bmrb.io).
1H and 13C spectra analyses
Individual free induction decay (.fid) files were processed using NMRPipe42, nmrglue43 and custom Python scripts available in the GitHub repository. Fourier transformed spectra were normalized by the noise root-mean-square error of the sparse 130–160 ppm region (Supplementary Figs. 2–4). The normalized spectrum stack was rendered as a surface plot in MATLAB R2019b (MathWorks) with face lightness mapped to the log2 of signal. Peaks with height ≥6 times the noise root-mean-square error (130–160 ppm) and separated from other peaks by 0.08 ppm were classified as detectable signals and assigned to compounds using the following algorithm:
-
(1)
Subpeaks within 0.3 ppm of each other were clustered.
-
(2)
Reference shifts for expected compounds within 0.45 ppm of the cluster were associated with the cluster.
-
(3)
All subpeaks from a cluster that associated with a single reference peak were assigned to the compound producing that reference peak.
-
(4)
In cases where a single cluster was associated with multiple reference peaks, subpeaks were manually assigned to compounds according to the reference chemical shifts from HMDB and associated splitting patterns. When the number of detected subpeaks was less than the expected multiplicity of the contributing reference peaks, extensive overlap was suspected and the clusters were excluded from analyses, as was the case for the isovalerate and isocaproate shifts at roughly 24.6 ppm in the [U-13C]leucine experiment. The remainder of manually assigned clusters used the 1D 13C and 1D 1H-13C HSQC spectra to deconvolute subpeaks in cases with resolvable overlap.
Assigned peak signals were concatenated into signal ridges, color-labeled by metabolite and superimposed over the stack as a scatter plot with stems to the xy plane and a smoothing spline curve fit. Surface regions within 0.5 ppm of each reference peak were colorized.
Assigned peaks in the 13C spectra ≤100 ppm were curve fit using a Voigt (Gaussian–Lorentzian convolution) lineshape and integrated. Logistic curve fits of metabolite integral versus time were calculated in Python using a least-squares regression (SciPy44 v.1.6.2) according to equation (1) (Supplementary Table 2). The peaks assigned to the acetate and ethanol methyl hydrogens in the 1H spectra from the [U-13C]glucose experiment were also curve fit to estimate the production of natural-abundance acetate.
Selenium perturbation experiment
HRMAS NMR was performed as described above with cultures in MMM containing an excess of 30 mM l-[U-13C]proline, with or without 100 µM sodium selenite. Proline depletion was estimated as the ratio of final integrated [13C]proline signal to the starting integrated [13C]proline signal. Relative 5-aminovalerate production was estimated by comparting the ratios of final integrated [13C]5-aminovalerate signal to the starting integrated [U-13C]proline signal.
Metabolic modeling
A previously published genome-scale metabolic model of C. difficile strain 630, icdf834 (ref. 45), was modified using the COBRApy toolbox46 and custom Python scripts. For metabolites not constrained by NMR trajectories, exchange reaction bounds were set to 3% of the millimolar concentration of media components used experimentally (Strain culture conditions). The cysteine uptake upper bound was set to 1,000 to enable uptake according to cellular demand. All other exchange reactions were blocked by setting their flux upper and lower bounds to zero.
icdf843, an updated model included in the GitHub repository, added the changes noted in Supplementary Table 4 (refs. 5,9,20,28,35), as supported by experimental data, and supported biologically relevant processes energetically and thermodynamically. Additions also included an exchange reaction for iron(II); transport and secretion reactions for propionate, phenylacetate, indole-3-acetate, butyrate, n-butanol and hydrogen sulfide; and proton-motive force dependent transport reactions for acetate, l-alanine, l-leucine, l-proline, l-isoleucine, isovalerate, isobutyrate, 2-methylbutyrate, isocaproate and 5-aminovalerate.
Estimation of exchange fluxes for dFBA
While the direct relationship between relative proton abundance and peak area in the 1H-NMR spectrum can be leveraged to estimate the relative abundance of protons at specific molecular contexts in a sample, an equivalent relationship between 13C abundance and peak area does not exist in 13C NMR due to molecular context effects, including NOE cross-relaxation between adjacent protons and the 13C nuclei21. This property of 13C NMR prevents credible estimates of metabolite concentrations using relative signal intensities alone.
To overcome limitations in 13C NMR for estimating metabolite concentrations to constrain exchange fluxes, the following approaches were used. First, the 13C substrate’s logistic derivative equation (3) was scaled by its input concentration in MMM, resulting in an equation for estimated uptake flux of the substrate. We next evaluated the metabolites produced during a labeled substrate’s fermentation and their predicted origin(s) from single or multiple metabolic pathways. If pathway associations were unknown or incompletely defined, the more complex case of multiple pathway origins was used.
The simplest approach, used in cases where a 1:1 relationship between the labeled substrate and its product eliminates the need for a product flux constraint, estimates substrate exchange flux using a logistic equation scaled by a known input substrate concentration. In C. difficile, proline fermentation is well-defined and is only known to follow a single pathway, yielding 5-aminovalerate4, making it an ideal candidate for this approach. A small amount of proline is also used for protein synthesis. Given the known concentration of proline in MMM, we transformed the logistic derivative equation (2) for proline into a flux trajectory by multiplying it by the factor [Proline]init/(LProline + CProline), where [Proline]init is the initial proline concentration in the NMR run and LProline and CProline are logistic equation (1) coefficients for proline. We left the 5-aminovalerate flux unconstrained as Stickland fermenters carrying a proline reductase consume most proline through this metabolic pathway4,25, with relatively modest proline use for biomass production. This approach performs best for compounds such as proline that possess a single, well-defined fermentation pathway, removing the need for a constraint on the product’s exchange flux.
In cases where a labeled substrate is metabolized into multiple products, such as leucine’s fermentation via two separate pathways5,6, we estimated exchange fluxes for the products isocaproate and isovalerate by determining an expected ratio using GC with flame ionization detection. Volatile short-chain fatty acids were extracted and quantified from stationary phase cultures of C. difficile ATCC 43255 ΔPaLoc grown in MMM lacking isoleucine as described in Girinathan et al.9, as shown in Supplementary Table 12. Expected isocaproate and isovalerate yields (YProduct) per mole of leucine were estimated by taking the molar ratio of each product in the flame ionization detection readout to the input leucine. Flux trajectories for the NMR runs were then estimated by multiplying equation (2) for each product by the factor [Leucine]init × YProduct/LProduct, where [Leucine]init is the initial leucine concentration in the NMR run.
In the third example, we estimated exchange fluxes of [U-13C]glucose metabolites using the input glucose concentration, the relative 13C NMR peak areas of glucose and each metabolite, and correction factors for NOE-induced signal enhancement empirically derived from standard solutions containing U-13C metabolites. Since glucose fermentation is highly integrated with central carbon metabolism, methods to estimate product concentration specific to the 13C input substrate are required, such as acetate originating from glucose versus glycine fermentation. For this case, we measured standard solutions within the dynamic range of our experiments under 13C NMR and integrated the peak areas to estimate a relationship between concentration and signal amplitude for each compound. As discussed in the Results section Dynamic FBA, 13C NMR signal amplitude is dependent on the molecular context of an individual 13C atom and is influenced by predictable factors, the most salient being 1H-13C NOE and spin rotation21. We assume that the relative effects of these properties on 13C NMR signal amplitude remain consistent between 13C atoms regardless of NMR acquisition parameters, and thus we define 13C NMR signal enhancement as the relative NMR signal-concentration ratio of each product with respect to [U-13C]glucose. Solutions listed in Supplementary Table 13 were measured by 13C NMR and processed using the procedures in HRMAS NMR and 1H and 13C spectra analyses above. Concentration-to-signal ratios of [U-13C]acetate, [U-13C]alanine, [U-13C]ethanol and [U-13C]butyrate (Sigma-Aldrich) in each standard solution were plotted versus the concentration-to-signal ratio of [U-13C]glucose in the same standard solution, then fit using a least-squares regression to the equation:
where 1/a represents the 13C NMR signal enhancement of each metabolite with respect to glucose (Extended Data Fig. 7). When low-intensity peaks were excluded (peak area <18 normalized signal units per ppm), a strong correlation was observed for each compound (R2 values displayed on Extended Data Fig. 7), suggesting that the 13C NMR signal enhancement of each metabolite with respect to glucose is consistent across experiments, even under inconsistent acquisition parameters. A normalization scheme for each product was then derived as follows:
where [Product]est is the estimated concentration of the product; [Glucose] is the known input concentration of [U-13C]glucose for the run; SProduct/SGlucose is the ratio of maximum integrated signal between the product and glucose, determined by fProduct(36)/fGlucose(0) where f is the logistic equation (1) and a is the product-specific coefficient in equation (3). The logistic derivative equation (2) for each product was then multiplied by [Product]est/LProduct and the logistic derivative equation (2) for glucose was multiplied by [Glucose]/(LGlucose + CGlucose) to transform the equations into flux trajectories. This approach is most suitable for compounds with multiple products where labeled products are commercially available or easily extracted.
In 1H-NMR, the multiplicity of protons can be estimated directly from the ratios of integrated signals. To estimate concentration and flux curves for natural-abundance acetate in the [U-13C]glucose run, the estimated concentration of 13C-acetate was multiplied by the ratio of integrated methyl proton signal between natural-abundance acetate and 13C-acetate.
The error in concentration and flux estimates was estimated by propagating the errors in the logistic coefficients as determined by the SciPy least-squares fit function. The function for standard error of the logistic equation (1) was defined as:
where:
and 𝛿L, 𝛿k, 𝛿x0 and 𝛿C are the standard errors of the logistic coefficients. Likewise, the function for standard error of the logistic derivative equation (2) was defined as:
where:
Aligning the time axes and calculating average logistic coefficients
The rate of isocaproate formation, as represented by the 0.864 ppm peak in the 1H-NMR time series, was selected as a normalization marker to align the time axes of all three runs because the 0.864 ppm isocaproate peak is well-separated in the 1H-NMR spectra, permitting reliable detection. A logistic curve (1) was fit to the 0.864 ppm isocaproate peak in the 1H time series of each run as described in the section 1H and 13C spectra analyses. The time axes for the three runs were normalized by aligning the time at which the logistic curve fit to the isocaproate 1H signal was 5% of its maximum value (Supplementary Figs. 1c,f,i, 2c,f,i and 3c,f,i).
Average logistic coefficients for all constrained metabolites were computed and averaged across three experimental replicates. Logistic coefficient errors were propagated as the root sum of squares (Supplementary Table 3). In cases where metabolites were not detected or signal was too low in a given run for successful logistic curve fitting, concentration estimates were scaled proportionally to the number of runs with signal supporting curve fitting.
Dynamic FBA
dFBA was implemented by computing steady-state FBA and FVA solutions with time-dependent exchange fluxes using the COBRApy toolbox46 and custom Python scripts. Exchange (for input metabolites) and secretion (for end metabolites) upper and lower bounds were set to the logistic function derivatives (2) transformed as explained in the sections Estimation of exchange fluxes for dFBA and Aligning the time axes and calculating average logistic coefficients, evaluated per time point (Fig. 2b,d,f and Supplementary Tables 4 and 6). Leucine exchange flux was left unconstrained due to low model tolerances. Roughly 39% of glucose consumption was not accounted for in the formation of short-chain fatty acids and alcohols and was likely diverted to the production of biomass or exopolysaccharide, in agreement with previously reported results from Dannheim et al. The glucose exchange fluxes were therefore limited to allow only uptake of glucose at a rate expected to yield the observed short-chain products. Glucose shunted to anabolic processes was not included due to competition of these processes with the ATP hydrolysis objective.
Before simulation, additional constraints were placed on the model. Neumann-Schaal et al. demonstrate that in amino acid-rich media, 2-methylbutyrate secretion roughly coincides with 5-aminovalerate secretion and isobutyrate secretion roughly cooccurs with isocaproate secretion23. Thus, the secretion flux of 2-methylbutyrate was set to the isovalerate trajectory, shifted such that the half-maximum coefficient x0 equaled that of 5-aminovalerate, and scaled such that the upper asymptote L equaled the input isoleucine concentration, assuming complete fermentation. Likewise, the secretion flux of isobutyrate was locked to the isovalerate trajectory, shifted such that the half-maximum coefficient x0 equaled that of isocaproate and scaled by the GC-determined isobutyrate/isovalerate ratio (Supplementary Table 12). Additionally, acetyl-CoA synthase flux was locked to the secretion of butyrate, according to in vitro results and the metabolic mechanism proposed by Gencic et al.35. Last, the 1H-NMR derived curve for natural-abundance acetate flux was added to the 13C NMR curve for glucose-derived acetate, to account for acetate-forming reactions from nonglucose carbon sources, including the fermentations of cysteine and glycine and the Wood–Ljungdahl pathway.
ATP hydrolysis was selected for the biological objective as a common metabolic driver during log phase, stationary phase and sporulation (Supplementary Information). Steady-state FBA solutions were calculated along a simulated 36-h timescale with a resolution of five solutions per hour. FVA solutions were computed along the same simulated timescale with an objective flux threshold of 99.5%. Metabolic flux trajectories of selected reactions were visualized using custom MATLAB scripts (Fig. 3). Fluxes contributing to the production and consumption of cytosolic l-alanine, l-glutamate, ATP, pyruvate and ammonia were recorded at each timepoint (Supplementary Table 6, columns C-AL).
Calculating flux proportions
Contributions to ATP influx were calculated by taking recorded reaction fluxes in Supplementary Table 6 as proportions of ATP flux as a whole:
-
1.
The early-phase ATP-producing Oxidative Stickland flux was taken as the ratio of ATP-producing oxidative Stickland flux from 0 to 9.8 h (dark-green shading) to total ATP influx from 0 to 9.8 h (light- and dark-green shading).
-
2.
The late-phase ATP-producing butyrate flux was taken as the ratio of butyrate kinase flux from 24 to 36 h (dark-orange shading) to total ATP influx from 24 to 36 h (light- and dark-orange shading).
-
3.
The total glycolytic contribution to ATP production was calculated as the ratio of the red-boxed region to the total ATP influx from 0 to 36 h.
-
4.
The glycolytic contribution to ATP production at each interval as the glycolytic flux in each interval (intersect of red box and each shaded region) divided by each shaded region’s flux (green, purple and orange for 0–9.8, 10–23.8 and 24–36 h, respectively).
-
5.
The proportion of ATP production in each time interval was taken as the ratio of each shaded region’s flux (green, purple and orange for 0–9.8, 10–23.8 and 24–36 h, respectively) to total ATP influx.
Predicted amino nitrogen flow from l-leucine to l-alanine was estimated by the following scheme. We define two phases of ammonia metabolism: the deamination phase from 1 to 9 h where there is net secretion of ammonia, and the assimilation phase from 10 to 21 h where there is net uptake of ammonia. To accurately capture the l-alanine amino-group contribution from l-leucine at each timepoint, we considered amino groups transferred directly from leucine to alanine via glutamate, as well as leucine-origin ammonia released during the deamination phase and recycled onto alanine via glutamate during the assimilation phase.
We assume that the final step of leucine-alanine amino transfer is the transamination of glutamate and pyruvate by alanine transaminase. Therefore, at each timepoint, share of leucine-origin alanine is the proportion of leucine-origin nitrogen on glutamate multiplied by the total alanine transaminase flux. This share of leucine-origin glutamate consists of only glutamate produced by leucine transaminase during the deamination phase. During the assimilation phase, there is an additional component of glutamate synthesized by the reverse reaction of glutamate dehydrogenase from leucine-origin ammonia that was released during the deamination phase. This second component is estimated as the proportion of pooled ammonia that originated as leucine amino nitrogen multiplied by the glutamate dehydrogenase flux.
The total leucine-origin ammonia is estimated as the cumulative sum of leucine amino nitrogen that has been deaminated by the combined actions of leucine transaminase and the forward reaction of glutamate dehydrogenase. At each timepoint, this is the glutamate dehydrogenase flux multiplied by the leucine transaminase flux as a proportion of total glutamate influx. The proportion of pooled ammonia that originated as leucine amino nitrogen at the end of the deamination phase is estimated as the cumulative sum of leucine-origin ammonia divided by the total ammonia production during the deamination phase.
All of these calculations are recorded in Supplementary Table 6 under the leucine-alanine calculations heading.
Analyses of 15N-13C peak splitting in 13C NMR spectral analyses of 13C alanine
For detection of 15N in the 13C spectrum, triplicate cultures of C. difficile ATCC 43255 ΔPaLoc were grown anaerobically in MMM containing [U-13C]glucose and either natural-abundance leucine or [15N]leucine. Cultures were centrifuged 48 h after inoculation and supernatants were collected, filter-sterilized, lyophilized and reconstituted in D2O. NMR data measured with Bruker BioSpin (Bruker Corporation) were analyzed with NUTS, 2D Pro (Acorn NMR Inc.). The .fid files were processed using 0.2 Hz line-broadening and were zero-filled before Fourier transformation. After the Fourier transformation, resonance peaks were quantified using curve fitting with Lorentzian line shapes. Curve fitting was first performed globally to determine the average peak width across all fitted peaks. The curve was then refitted using the width average and Lorentzian function (Extended Data Fig. 5).
13C NMR analyses of alanine’s α-carbon in media with [U-13C]glucose and [15N]leucine
The complex peaks from alanine’s α-carbon in the 13C NMR spectral region can be deconvoluted based on (1) possible 13C label patterns (Extended Data Fig. 8a), where (a) 13C can appear on all three carbons in alanine that results in quadruple peaks occurring at the α-carbon; (b) carbon-13 can also appear on only two carbons in alanine: [1,2-13C]alanine and [2,3-13C]alanine, while [1,3-13C]alanine produces no detectable α-carbon-13C NMR signal and both two 13C-labeled isotopologs of alanine produce doublet peaks for the α-carbon and (c) carbon-13 only appears on the α-carbon as [2-13C]alanine to produce a singlet peak. (2) J-coupling of 15N-13C splits the α-carbon-13C NMR peak into two peaks when 15N is bound to α-13C (Extended Data Fig. 8b). (3) the isotope effect that introduces small shifts in the measured resonances33 (Extended Data Fig. 8d).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All NMR free induction decay files generated during the current study are available at the Metabolomics Workbench47 as study ST002433 (https://doi.org/10.21228/M88M5G). The updated C. difficile metabolic model icdf843 is available on GitHub at https://github.com/Massachusetts-Host-Microbiome-Center/nmr-cdiff. The remaining data generated in this study are included in this article and its supplementary information files. Reference spectra used in this study to identify molecules in the NMR spectra were accessed from the HMDB (https://hmdb.ca/) and BMRB (https://bmrb.io) for l-proline (HMDB, HMDB0000162; BMRB, bmse000047), 5-aminovalerate (HMDB, HMDB0003355; BMRB, bmse000419), l-leucine (HMDB, HMDB0000687; BMRB, bmse000042), isovalerate (HMDB, HMDB0000718; BMRB, bmse000373), isocaproate (HMDB, HMDB0000689), d-glucose (HMDB, HMDB0000122; BMRB, bmse000015), acetate (HMDB, HMDB0000042; BMRB, bmse000191), ethanol (HMDB, HMDB0000108; BMRB, bmse000297), l-alanine (HMDB, HMDB0000161; BMRB, bmse000994), l-lactate (HMDB, HMDB0000190; BMRB, bmse000269), butyrate (HMDB, HMDB0000039; BMRB, bmse000402) and n-butanol (HMDB, HMDB0004327; BMRB, bmse000447).
Code availability
All custom code developed for this study is available on GitHub at https://github.com/Massachusetts-Host-Microbiome-Center/nmr-cdiff.
References
Schonheit, P., Buckel, W. & Martin, W. F. On the origin of heterotrophy. Trends Microbiol 24, 12–25 (2016).
Cheng, C., Bao, T. & Yang, S. T. Engineering Clostridium for improved solvent production: recent progress and perspective. Appl. Microbiol. Biotechnol. 103, 5549–5566 (2019).
Guh, A. Y. et al. Trends in U.S. burden of Clostridioides difficile infection and outcomes. N. Engl. J. Med. 382, 1320–1330 (2020).
Bouillaut, L., Self, W. T. & Sonenshein, A. L. Proline-dependent regulation of Clostridium difficile Stickland metabolism. J. Bacteriol. 195, 844–854 (2013).
Kim, J., Darley, D., Selmer, T. & Buckel, W. Characterization of (R)-2-hydroxyisocaproate dehydrogenase and a family III coenzyme A transferase involved in reduction of l-leucine to isocaproate by Clostridium difficile. Appl. Environ. Microbiol. 72, 6062–6069 (2006).
Pavao, A. et al. Reconsidering the in vivo functions of Clostridial Stickland amino acid fermentations. Anaerobe https://doi.org/10.1016/j.anaerobe.2022.102600 (2022).
Fletcher, J. R. et al. Clostridioides difficile exploits toxin-mediated inflammation to alter the host nutritional landscape and exclude competitors from the gut microbiota. Nat. Commun. 12, 462 (2021).
Bouillaut, L., Dubois, T., Sonenshein, A. L. & Dupuy, B. Integration of metabolism and virulence in Clostridium difficile. Res. Microbiol. 166, 375–383 (2015).
Girinathan, B. P. et al. In vivo commensal control of Clostridioides difficile virulence. Cell Host Microbe 29, 1693–1708.e1697 (2021).
Cheng, L. L. et al. Quantitative neuropathology by high resolution magic angle spinning proton magnetic resonance spectroscopy. Proc. Natl Acad. Sci. USA 94, 6408–6413 (1997).
Tilgner, M., Vater, T. S., Habbel, P. & Cheng, L. L. High-resolution magic angle spinning (HRMAS) NMR methods in metabolomics. Methods Mol. Biol. 2037, 49–67 (2019).
Judge, M. T. et al. Continuous in vivo metabolism by NMR. Front Mol. Biosci. 6, 26 (2019).
Cheng, L. L. et al. Enhanced resolution of proton NMR spectra of malignant lymph nodes using magic-angle spinning. Magn. Reson. Med. 36, 653–658 (1996).
Henry, P. G. et al. In vivo 13C NMR spectroscopy and metabolic modeling in the brain: a practical perspective. Magn. Reson. Imaging 24, 527–539 (2006).
Rados, D. et al. Carbon flux analysis by 13C nuclear magnetic resonance to determine the effect of CO2 on anaerobic succinate production by Corynebacterium glutamicum. Appl. Environ. Microbiol. 80, 3015–3024 (2014).
Zamboni, N., Fendt, S. M., Ruhl, M. & Sauer, U. 13C-based metabolic flux analysis. Nat. Protoc. 4, 878–892 (2009).
Borah Slater, K. et al. One-shot 13C15N-metabolic flux analysis for simultaneous quantification of carbon and nitrogen flux. Mol. Syst. Biol. https://doi.org/10.15252/msb.202211099 (2023).
Mahadevan, R., Edwards, J. S. & Doyle, F. J. III. Dynamic flux balance analysis of diauxic growth in Escherichia coli. Biophys. J. 83, 1331–1340 (2002).
Henson, M. A. & Hanly, T. J. Dynamic flux balance analysis for synthetic microbial communities. IET Syst. Biol. 8, 214–229 (2014).
Dannheim, H., Will, S. E., Schomburg, D. & Neumann-Schaal, M. Clostridioides difficile 630Deltaerm in silico and in vivo—quantitative growth and extensive polysaccharide secretion. FEBS Open Bio. 7, 602–615 (2017).
Becker, E. D., Shoup, R. R. & Farrar, T. C. 13C NMR spectroscopy: relaxation times of 13C and methods for sensitivity enhancement. Pure Appl. Chem. 32, 51–66 (2009).
Cheng, L. L. High-resolution magic angle spinning NMR for intact biological specimen analysis: Initial discovery, recent developments, and future directions. NMR Biomed. https://doi.org/10.1002/nbm.4684 (2021).
Neumann-Schaal, M., Hofmann, J. D., Will, S. E. & Schomburg, D. Time-resolved amino acid uptake of Clostridium difficile 630Deltaerm and concomitant fermentation product and toxin formation. BMC Microbiol. 15, 281 (2015).
Britz, M. L. & Wilkinson, R. G. Leucine dissimilation to isovaleric and isocaproic acids by cell suspensions of amino acid fermenting anaerobes: the Stickland reaction revisited. Can. J. Microbiol. 28, 291–300 (1982).
Jackson, S., Calos, M., Myers, A. & Self, W. T. Analysis of proline reduction in the nosocomial pathogen Clostridium difficile. J. Bacteriol. 188, 8487–8495 (2006).
Lovitt, R. W., Kell, D. B. & Morris, J. G. Proline reduction by Clostridium sporogenes is coupled to vectorial proton ejection. FEMS Microbiol. Lett. 36, 269–273 (1986).
Rennhack, K. E. The Protein Network of the Rnf-Proline Reductase Complex Required for Respiratory Energy Generation in Clostridioides difficile. PhD thesis, Technische Univ. Braunschweig (2021).
Neumann-Schaal, M., Jahn, D. & Schmidt-Hohagen, K. Metabolism the difficile way: the key to the success of the pathogen Clostridioides difficile. Front Microbiol 10, 219 (2019).
Kabisch, U. C. et al. Identification of d-proline reductase from Clostridium sticklandii as a selenoenzyme and indications for a catalytically active pyruvoyl group derived from a cysteine residue by cleavage of a proprotein. J. Biol. Chem. 274, 8445–8454 (1999).
Patching, S. G. NMR-active nuclei for biological and biomedical applications. J. Diagnostic Imaging Ther. 3, 7–48 (2016).
Nieto, R., Cruz, F., Tejedor, J. M., Barroso, G. & Cerdan, S. Origin of the ammonia used for mitochondrial citrulline synthesis as revealed by 13C-15N spin coupling patterns observed by 13C NMR. Biochimie 74, 903–911 (1992).
Lapidot, A. & Gopher, A. Quantitation of metabolic compartmentation in hyperammonemic brain by natural abundance 13C-NMR detection of 13C-15N coupling patterns and isotopic shifts. Eur. J. Biochem. 243, 597–604 (1997).
Lambert, J. B. & Greifenstein, L. G. Origin of the chemical-shift isotope effect. Stereochemical evidence. J. Am. Chem. Soc. 96, 5120–5124 (1974).
Ragsdale, S. W. Enzymology of the Wood–Ljungdahl pathway of acetogenesis. Ann. N. Y. Acad. Sci. 1125, 129–136 (2008).
Gencic, S. & Grahame, D. A. Diverse energy-conserving pathways in Clostridium difficile: growth in the absence of amino acid Stickland acceptors and the role of the Wood–Ljungdahl pathway. J. Bacteriol. https://doi.org/10.1128/JB.00233-20 (2020).
Peltier, J. et al. Genomic and expression analysis of the vanG-like gene cluster of Clostridium difficile. Microbiol. 159, 1510–1520 (2013).
Hofmann, J. D. et al. Metabolic reprogramming of Clostridioides difficile during the stationary phase with the induction of toxin production. Front. Microbiol. 9, 1970 (2018).
Philips, J., Rabaey, K., Lovley, D. R. & Vargas, M. Biofilm formation by Clostridium ljungdahlii is induced by sodium chloride stress: experimental evaluation and transcriptome analysis. PLoS ONE 12, e0170406 (2017).
Katsube, S., Sato, K., Ando, T., Isogai, E. & Yoneyama, H. Secretion of d-alanine by Escherichia coli. Microbiol. 162, 1243–1252 (2016).
Peltier, J. et al. Type I toxin-antitoxin systems contribute to the maintenance of mobile genetic elements in Clostridioides difficile. Commun. Biol. 3, 718 (2020).
Karasawa, T., Ikoma, S., Yamakawa, K. & Nakamura, S. A defined growth medium for Clostridium difficile. Microbiology 141, 371–375 (1995).
Delaglio, F. et al. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J. Biomol. NMR 6, 277–293 (1995).
Helmus, J. J. & Jaroniec, C. P. Nmrglue: an open source Python package for the analysis of multidimensional NMR data. J. Biomol. NMR 55, 355–367 (2013).
Virtanen, P. et al. SciPy 1.0: fundamental algorithms for scientific computing in Python. Nat. Methods 17, 261–272 (2020).
Kashaf, S. S., Angione, C. & Lio, P. Making life difficult for Clostridium difficile: augmenting the pathogen’s metabolic model with transcriptomic and codon usage data for better therapeutic target characterization. BMC Syst. Biol. 11, 25 (2017).
Ebrahim, A., Lerman, J. A., Palsson, B. O. & Hyduke, D. R. COBRApy: constraints-based reconstruction and analysis for Python. BMC Syst. Biol. 7, 74 (2013).
Sud, M. et al. Metabolomics Workbench: an international repository for metabolomics data and metadata, metabolite standards, protocols, tutorials and training, and analysis tools. Nucleic Acids Res. 44, D463–D470 (2016).
Acknowledgements
We thank M. Delaney for microbiology support, M. Judge and A. Edison for helpful comments and for confirming capacity of sealed HRMAS NMR rotors to maintain anaerobic conditions. This project was supported by the National Institutes of Health (L.B., grant nos. R01AI153653, R03AI174158 and P30DK056338; L.L.C., grant nos. S10OD023406, R21CA243255 and R01AG070257), the Brigham and Women’s Hospital Precision Medicine Institute and Presidential Scholar’s Award (L.B.), the MGH A. A. Martinos Center for Biomedical Imaging (L.B. and L.L.C.) and the Massachusetts Life Sciences Center (L.B. and L.L.C.).
Author information
Authors and Affiliations
Contributions
L.B. and L.L.C. conceived the study. J.P., P.A.S. and B.D. constructed the PaLoc-negative strain. A.P., B.G. and L.B. developed the anaerobic microbiology methods and performed the microbiological components of the HRMAS experiments. L.L.C. developed the NMR methodologies and performed the magnetic resonance spectroscopys components of the HRMAS experiments. A.P., I.H.M. and L.L.C. processed and analyzed the NMR data. C.M. modeled and advised on the technique for 15N-13C amplification. A.P. developed the custom code and performed the computational and metabolic modeling analyses. A.P. and L.B. wrote the original draft. L.L.C., J.P., P.A.S., B.D., I.H.M. and C.M. provided edits and content to the original draft.
Corresponding authors
Ethics declarations
Competing interests
L.B. is the inventor of live bacteriotherapeutics for C. difficile, the scientific founder, SAB chair and a stockholder in ParetoBio, Inc. The other authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks William T. Self, Alexander Shekhtman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Metabolic consistency of HRMAS acquisition with standard anaerobic cultures in Kel-F rotor inserts.
Analyses of C. difficile cultures grown in MMM in a sealed Kel-F rotor insert, either incubated for 36 h at 37 °C without spinning (“nonspinning”) or subjected to the HRMAS radiofrequency pulse and spinning program for 36 h (“HRMAS”). (a–b) Optical microscopy images of Gram-stained cultures grown in the (a) non-spinning or (b) spinning condition; each microscopy image represents three biological replicates yielding similar results. Colored arrows indicate vegetative cells (black arrows), lysed cells and debris (blue arrows), and sporulating cells (red arrows). (c–d) 1H-NMR spectra of cultures grown in MMM with [U-13C]proline in the (c) non-spinning or (d) HRMAS condition. Axes: unitless NMR signal (y axis) versus chemical shift in ppm (x axis). Labels identify 1H peaks attributed to selected metabolites. (e–f) Cultures grown in MMM with [U-13C]leucine in the (e) non-spinning or (f) HRMAS condition; axes and labels as in (c–d). (g–h) Cultures grown in MMM with [U-13C]glucose in the (g) non-spinning or (h) HRMAS condition; axes and labels as in (c–d).
Extended Data Fig. 2 HRMAS 13C NMR of C. difficile grown with L-[U-13C]proline.
(a) Surface plot of 13C-NMR time series for experimental replicate 1 with trajectories of peaks attributed to proline (dark blue) and 5-aminovalerate (dark red). Axes: unitless NMR signal (z axis) versus chemical shift in ppm (x axis) versus time in hours (y axis). (b) Scatter plot for experimental replicate 1 of estimated metabolite concentrations (mM) with logistic curve fit versus time in hours. (c) Surface plot of 13C-NMR time series for experimental replicate 2. Lines and axes as in (a). (d) Scatter plot for experimental replicate 2 of estimated metabolite concentrations, axes as in (b). (e) Surface plot of 13C-NMR time series for experimental replicate 3. Lines and axes as in (a). (f) Scatter plot for experimental replicate 3 of estimated metabolite concentrations, axes as in (b).
Extended Data Fig. 3 HRMAS 13C NMR of C. difficile grown with L-[U-13C]leucine.
(a) Surface plot of 13C-NMR time series for experimental replicate 1 with trajectories of peaks attributed to leucine (teal) and its fermentation products. Axes: unitless NMR signal (z axis) versus chemical shift in ppm (x axis) versus time in hours (y axis). (b) Scatter plot for experimental replicate 1 of estimated metabolite concentrations (mM) with logistic curve fit versus time in hours. (c) Surface plot of 13C-NMR time series for experimental replicate 2. Lines and axes as in (a). (d) Scatter plot for experimental replicate 2 of estimated metabolite concentrations, axes as in (b). [U-13C]Isovalerate in this run lacked sufficient signal to infer a credible trajectory. (e) Surface plot of 13C-NMR time series for experimental replicate 3. Lines and axes as in (a). (f) Scatter plot for experimental replicate 3 of estimated metabolite concentrations, axes as in (b).
Extended Data Fig. 4 HRMAS 13C NMR of C. difficile grown with [U-13C]glucose.
(a) Surface plot of 13C-NMR time series for experimental replicate 1 with trajectories of peaks attributed to glucose (blue) and its fermentation products. Axes: unitless NMR signal (z axis) versus chemical shift in ppm (x axis) versus time in hours (y axis). (b) Scatter plot for experimental replicate 1 of estimated metabolite concentrations (mM) with logistic curve fit versus time in hours. (c) Surface plot of 13C-NMR time series for experimental replicate 2. Lines and axes as in (a). (d) Scatter plot for experimental replicate 2 of estimated metabolite concentrations, axes as in (b). (e) Surface plot of 13C-NMR time series for experimental replicate 3. Lines and axes as in (a). (f) Scatter plot for experimental replicate 3 of estimated metabolite concentrations, axes as in (b).
Extended Data Fig. 5 Curve-fitted 13C NMR spectral region of alanine’s α-carbon following the growth of live C. difficile in [U-13C]glucose with natural abundance leucine or [15N]leucine.
(a) Spectrum of culture grown in media containing natural abundance leucine. (b) Spectrum of culture grown in media containing [15N]leucine. In each resonance spectrum, blue indicates the curve of the experimental raw data; red indicates the overall fitted curve; and green indicates the individual fitted peaks; the grey line indicates the difference between the experimental and fitted curves.
Extended Data Fig. 6 Live cell 13C NMR spectra and metabolite identification.
(a) Proton-decoupled 13C NMR spectra of live cells following reactions with [U-13C]glucose, L-[U-13C]proline, and L-[U-13C]leucine, respectively. (b) 1H and proton-decoupled 13C NMR spectra of [U-13C]glucose produced 13C2-acetate, measured at 45.5 and 45.0 h, respectively. Relative signal-to-noise ratio (S/N) normalized to unit time for measured for 1H (S/N = 1.000) and 13C (S/N = 0.033). The double-doublet peaks seen in the 1H spectrum resulted from 1H J-couplings with both methyl and carboxyl 13C nuclei. The center peak represented methyl-1H bond to 12C. The doublet peaks seen in the 13C spectrum were the result of 13C-13C J-coupling between methyl and carboxyl 13C nuclei. (c) An illustration of metabolite identifications for live cells grown with [U-13C]glucose through 2D 13C COSY, 13C-1H HSQC, and 1H COSY according to reported 13C and 1H chemical shift values from the HMDB. In this illustration, the 1H 1D spectrum was measured at 45.5 h; 1H 2D COSY, at 32.6 h; 1H-13C HSQC, at 27.9 h; 13C COSY, at 37.2 h; and 13C 1D spectrum was a composite spectrum of all proton-decoupled 13C spectra (N = 11) measured between 24.8 and 45.0 h.
Extended Data Fig. 7 13C-signal enhancement standard curves of U-13C metabolites with respect to glucose.
(a) Scatter plot of concentration-signal ratio of [U-13C]acetate versus [U-13C]glucose, determined from HRMAS 13C-NMR acquisitions of the solutions listed in Supplementary Table 9. Only peaks ≤ 100 ppm are included in the analyses; acquisitions where the average per-carbon peak area for a compound is less than 18 are marked with a black cross and excluded from the linear regression. Best fit line shown with equation and R-squared statistic. (b) Scatter plot of concentration-signal ratio of [U-13C]alanine versus [U-13C]glucose, determined as in panel (a). (c) Scatter plot of concentration-signal ratio of [U-13C]butyrate versus [U-13C]glucose, determined as in panel (a). (d) Scatter plot of concentration-signal ratio of [U-13C]ethanol versus [U-13C]glucose, determined as in panel (a).
Extended Data Fig. 8 Deconvolution of alanine’s α-carbon in the 13C NMR spectral region.
(a) All possible 13C patterns of alanine’s α-carbon, as produced from [U-13C]glucose, are shown in gray (Q: quadruple peaks from [U-13C]alanine, D12 and D23: doublets from [1,2-13C]alanine and [2,3-13C]alanine, respectively, S: singlet from [2-13C]alanine. (b) All peaks split into two equal intensity peaks due to 15N-13C J-coupling, shown in yellow. (c) The sum of peaks in A and B. (d) Predicted peak profile of all possible alanine species, with calculations that include shift of 15N associated 13C resonance due to isotope effects.
Supplementary information
Supplementary Information
Supplementary tables 1–3, 9 and 11–13 and figs. 1–4.
Supplementary Tables 4–8, 10
Supplementary Table 4: Modifications to icdf834, a previously published model of C. difficile28. The resulting model is named icdf843. Modifications are further elaborated in the Methods (Metabolic modeling). Supplementary Table 5: Evaluated exchange reaction bounds for NMR-measured metabolites. Uptake (proline, valine, isoleucine, and glucose) or secretion (all other metabolites) reaction upper and lower bounds as evaluated at each simulated timepoint, displayed in mM h−1. Supplementary Table 6: Selected dFBA-predicted metabolic fluxes. Predicted producing and consuming fluxes for l-alanine (alaL_c, producing: column C, consuming: column D), l-glutamate (gluL_c, producing: columns E-H, consuming: columns I-K), ATP (atp_c: columns L-S, consuming: columns T-Y), pyruvate (pyr_c: columns Z-AB, consuming: columns AC-AE), and ammonia (nh3_c: columns AF-AJ, consuming: columns AK-AL). The model objective flux is in column U. Calculations for estimated amino-group flux from leucine to alanine are in columns AM-BA. Supplementary Table 7: FVA simulation reaction lower bounds for all reactions in icdf843. Lower bounds for reactions accommodating an ATP hydrolysis objective flux within 0.5% of the optimal value. Flux units in mM h−1, table headers are reaction IDs. Supplementary Table 8: FVA simulation reaction upper bounds for all reactions in icdf843. Upper bounds for reactions accommodating an ATP hydrolysis objective flux within 0.5% of the optimal value. Flux units in mM h−1, table headers are reaction IDs. Supplementary Table 10: Pathways and enzyme systems involved in the metabolism of glucose, leucine, and proline in C. difficile. Column labels indicate the following information. Number, corresponding reaction numbers shown in Figs. 2 and 3. System or Enzyme, corresponding cellular system or enzyme-catalyzed pathway. Gene-association, CD630: associated geneID in the C. difficile reference strain CD630. Gene-association: ATCC43255, associated geneID in the C. difficile strain ATCC43255 used in the present studies.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pavao, A., Girinathan, B., Peltier, J. et al. Elucidating dynamic anaerobe metabolism with HRMAS 13C NMR and genome-scale modeling. Nat Chem Biol 19, 556–564 (2023). https://doi.org/10.1038/s41589-023-01275-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-023-01275-9
This article is cited by
-
Metabolism from the magic angle
Nature Chemical Biology (2023)