Abstract
Insulin and its related peptides are found throughout the animal kingdom, in which they serve diverse functions. This includes regulation of glucose homeostasis, neuronal development and cognition. The surprising recent discovery that venomous snails evolved specialized insulins to capture fish demonstrated the nefarious use of this hormone in nature. Because of their streamlined role in predation, these repurposed insulins exhibit unique characteristics that have unraveled new aspects of the chemical ecology and structural biology of this important hormone. Recently, insulins were also reported in other venomous predators and pathogenic viruses, demonstrating the broader use of insulin by one organism to manipulate the physiology of another. In this Review, we provide an overview of the discovery and biomedical application of repurposed insulins and other hormones found in nature and highlight several unique insights gained from these unusual compounds.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Zheng, S. et al. A functional study of all 40 Caenorhabditis elegans insulin-like peptides. J. Biol. Chem. 293, 16912–16922 (2018).
Wu, Q. & Brown, M. R. Signaling and function of insulin-like peptides in insects. Annu. Rev. Entomol. 51, 1–24 (2006).
Avruch, J. Insulin signal transduction through protein kinase cascades. Mol. Cell. Biochem. 182, 31–48 (1998).
Taniguchi, C. M., Emanuelli, B. & Kahn, C. R. Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 7, 85–96 (2006).
Menting, J. G. et al. Protective hinge in insulin opens to enable its receptor engagement. Proc. Natl Acad. Sci. USA 111, E3395–E3404 (2014).
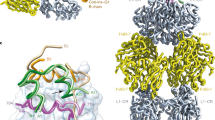
Menting, J. G. et al. How insulin engages its primary binding site on the insulin receptor. Nature 493, 241–245 (2013). Seminal study that revealed the structural interactions of insulin with the μIR, a domain-minimized version of the IR comprising the two-domain L1–CR receptor fragment and the αCT segment that form the receptor’s primary hormone-binding site.
Scapin, G. et al. Structure of the insulin receptor–insulin complex by single-particle cryo-EM analysis. Nature 556, 122–125 (2018). A cryo-EM structure of the IR–insulin complex.
Gutmann, T. et al. Cryo-EM structure of the complete and ligand-saturated insulin receptor ectodomain. J. Cell Biol. 219, e201907210 (2020).
Uchikawa, E., Choi, E., Shang, G., Yu, H. & Bai, X. C. Activation mechanism of the insulin receptor revealed by cryo-EM structure of the fully liganded receptor–ligand complex. eLife 8, e48630 (2019).
Weis, F. et al. The signalling conformation of the insulin receptor ectodomain. Nat. Commun. 9, 4420 (2018).
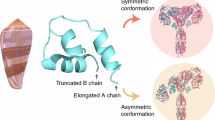
Xiong, X. et al. Symmetric and asymmetric receptor conformation continuum induced by a new insulin. Nat. Chem. Biol.18, 511–519 (2022).
Nielsen, J. et al. Structural investigations of full-length insulin receptor dynamics and signalling. J. Mol. Biol. 434, 167458 (2022).
Lawrence, M. C. Understanding insulin and its receptor from their three-dimensional structures. Mol. Metab. 52, 101255 (2021). Insightful review on the current understanding of how insulin engages with the IR. It also discusses important remaining questions in the field.
Blumenthal, S. From insulin and insulin-like activity to the insulin superfamily of growth-promoting peptides: a 20th-century odyssey. Perspect. Biol. Med. 53, 491–508 (2010).
Cherif-Feildel, M. et al. Molecular evolution and functional characterisation of insulin related peptides in molluscs: contributions of Crassostrea gigas genomic and transcriptomic-wide screening. Gen. Comp. Endocrinol. 271, 15–29 (2019).
Elphick, M. R., Mirabeau, O. & Larhammar, D. Evolution of neuropeptide signalling systems. J. Exp. Biol. 221, jeb151092 (2018).
Mack, L. R. & Tomich, P. G. Gestational diabetes: diagnosis, classification, and clinical care. Obstet. Gynecol. Clin. North Am. 44, 207–217 (2017).
Nkonge, K. M., Nkonge, D. K. & Nkonge, T. N. The epidemiology, molecular pathogenesis, diagnosis, and treatment of maturity-onset diabetes of the young (MODY). Clin. Diabetes Endocrinol. 6, 20 (2020).
Zaykov, A. N., Mayer, J. P. & DiMarchi, R. D. Pursuit of a perfect insulin. Nat. Rev. Drug Discov. 15, 425–439 (2016).
Beardwood, J. T. A case of attempted suicide with insulin. J. Am. Med. Assoc. 102, 765–766 (1943).
Marks, V. Murder by insulin: suspected, purported and proven—a review. Drug Test. Anal. 1, 162–176 (2009).
Marks, V. & Richmond, C. Insulin Murders: True Life Cases 1st edn (RSM, 2007).
Nelson, J., Ibrahim, J., Bugeja, L. & Ranson, D. Killing of elderly patients by health care professionals: insights from coroners’ inquests and inquiries in three cases. J. Law Med. 28, 620–631 (2021).
Geppert, C. The angel of death in Clarksburg. Fed. Practitioner 38, 564–565 (2021).
Safavi-Hemami, H. et al. Specialized insulin is used for chemical warfare by fish-hunting cone snails. Proc. Natl Acad. Sci. USA 112, 1743–1748 (2015). Discovery of the use of insulin as a weapon for prey capture by venomous marine cone snails.
Johnson, C. R. & Stablum, W. Observations on the feeding behavior of Conus geographus (Gastropoda: Toxoglossa). Pac. Sci. 25, 109–111 (1971).
Aman, J. W. et al. Insights into the origins of fish hunting in venomous cone snails from studies of Conus tessulatus. Proc. Natl Acad. Sci. USA 112, 5087–5092 (2015).
Bingham, J. P., Baker, M. R. & Chun, J. B. Analysis of a cone snail’s killer cocktail—the milked venom of Conus geographus. Toxicon 60, 1166–1170 (2012).
Menting, J. G. et al. A minimized human insulin-receptor-binding motif revealed in a Conus geographus venom insulin. Nat. Struct. Mol. Biol. 23, 916–920 (2016).
Gradel, A. K. J. et al. Factors affecting the absorption of subcutaneously administered insulin: effect on variability. J. Diabetes Res. 2018, 1205121 (2018).
Heinemann, L. Variability of insulin absorption and insulin action. Diabetes Technol. Ther. 4, 673–682 (2004).
Carpenter, F. H. Relationship of structure to biological activity of insulin as revealed by degradative studies. Am. J. Med. 40, 750–758 (1966).
Xiong, X. et al. A structurally minimized yet fully active insulin based on cone-snail venom insulin principles. Nat. Struct. Mol. Biol. 27, 615–624 (2020). This study demonstrates that an insulin from cone snail venom could inform on the design of a monomeric insulin analog as a potential therapeutic for diabetes.
De Meyts, P. Structural basis for the poisonous activity of a predator’s venom insulin. Nat. Struct. Mol. Biol. 23, 872–874 (2016).
Lu, A. et al. Transcriptomic profiling reveals extraordinary diversity of venom peptides in unexplored predatory gastropods of the genus Clavus. Genome Biol. Evol. 12, 684–700 (2020).
Fassio, G. et al. Venom diversity and evolution in the most divergent cone snail genus Profundiconus. Toxins 11, 623 (2019).
Pardos-Blas, J. R. et al. The genome of the venomous snail Lautoconus ventricosus sheds light on the origin of conotoxin diversity. GigaScience 10, giab037 (2021).
Safavi-Hemami, H. et al. Venom insulins of cone snails diversify rapidly and track prey taxa. Mol. Biol. Evol. 33, 2924–2934 (2016).
Ahorukomeye, P. et al. Fish-hunting cone snail venoms are a rich source of minimized ligands of the vertebrate insulin receptor. eLife 8, e41574 (2019).
Conlon, M. Molecular evolution of insulin in non-mammalian vertebrates. Am. Zool. 40, 200–212 (2000).
Jiracek, J. & Zakova, L. From venom peptides to a potential diabetes treatment. eLife 8, e44829 (2019).
Vonk, F. J. et al. The king cobra genome reveals dynamic gene evolution and adaptation in the snake venom system. Proc. Natl Acad. Sci. USA 110, 20651–20656 (2013).
Sparkman, A. M. et al. Rates of molecular evolution vary in vertebrates for insulin-like growth factor-1 (IGF-1), a pleiotropic locus that regulates life history traits. Gen. Comp. Endocrinol. 178, 164–173 (2012).
Kunalan, S., Othman, I., Syed Hassan, S. & Hodgson, W. C. Proteomic characterization of two medically important Malaysian snake venoms, Calloselasma rhodostoma (Malayan pit viper) and Ophiophagus hannah (king cobra). Toxins 10, 434 (2018).
Tan, C. H., Tan, K. Y., Fung, S. Y. & Tan, N. H. Venom-gland transcriptome and venom proteome of the Malaysian king cobra (Ophiophagus hannah). BMC Genomics 16, 687 (2015).
Danpaiboon, W. et al. Ophiophagus hannah venom: proteome, components bound by Naja kaouthia antivenin and neutralization by N. kaouthia neurotoxin-specific human ScFv. Toxins 6, 1526–1558 (2014).
Hu, H., Bandyopadhyay, P. K., Olivera, B. M. & Yandell, M. Elucidation of the molecular envenomation strategy of the cone snail Conus geographus through transcriptome sequencing of its venom duct. BMC Genomics 13, 284 (2012).
McCullough, D., Atofanei, C., Knight, E., Trim, S. A. & Trim, C. M. Kinome scale profiling of venom effects on cancer cells reveals potential new venom activities. Toxicon 185, 129–146 (2020).
Altindis, E. et al. Viral insulin-like peptides activate human insulin and IGF-1 receptor signaling: a paradigm shift for host–microbe interactions. Proc. Natl Acad. Sci. USA 115, 2461–2466 (2018). Discovery that viruses encode insulin-like peptides that can activate human insulin and IGF-1 receptor signaling.
Chrudinová, M. et al. Characterization of viral insulins reveals white adipose tissue-specific effects in mice. Mol. Metab. 44, 101121 (2021).
Zhang, F., Altindis, E., Kahn, C. R., DiMarchi, R. D. & Gelfanov, V. A viral insulin-like peptide is a natural competitive antagonist of the human IGF-1 receptor. Mol. Metab. 53, 101316 (2021).
Huang, Q., Kahn, C. R. & Altindis, E. Viral hormones: expanding dimensions in endocrinology. Endocrinology 160, 2165–2179 (2019).
Girdhar, K. et al. Viruses and metabolism: the effects of viral infections and viral insulins on host metabolism. Annu. Rev. Virol. 8, 373–391 (2021).
Nadkarni, P., Chepurny, O. G. & Holz, G. G. Regulation of glucose homeostasis by GLP-1. Prog. Mol. Biol. Transl. Sci. 121, 23–65 (2014).
Mentlein, R., Gallwitz, B. & Schmidt, W. E. Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum. Eur. J. Biochem. 214, 829–835 (1993).
Tsend-Ayush, E. et al. Monotreme glucagon-like peptide-1 in venom and gut: one gene—two very different functions. Sci. Rep. 6, 37744 (2016).
Wong, E. S., Nicol, S., Warren, W. C. & Belov, K. Echidna venom gland transcriptome provides insights into the evolution of monotreme venom. PLoS ONE 8, e79092 (2013).
Xu, X. & Lai, R. The chemistry and biological activities of peptides from amphibian skin secretions. Chem. Rev. 115, 1760–1846 (2015).
Montecucchi, P. C., de Castiglione, R., Piani, S., Gozzini, L. & Erspamer, V. Amino acid composition and sequence of dermorphin, a novel opiate-like peptide from the skin of Phyllomedusa sauvagei. Int. J. Pept. Protein Res. 17, 275–283 (1981).
Hesselink, J. M. K. & Schatman, M. E. Rediscovery of old drugs: the forgotten case of dermorphin for postoperative pain and palliation. J. Pain. Res. 11, 2991–2995 (2018).
Anastasi, A., Erspamer, V. & Bucci, M. Isolation and structure of bombesin and alytesin, 2 analogous active peptides from the skin of the European amphibians Bombina and Alytes. Experientia 27, 166–167 (1971).
Ladenheim, E. E. in Handbook of Biologically Active Peptides (ed. Kastin, A. J.) Ch. 142, 1064–1070 (Academic, 2013).
Bordon, K. C. F. et al. From animal poisons and venoms to medicines: achievements, challenges and perspectives in drug discovery. Front. Pharmacol. 11, 1132 (2020).
Schweitz, H., Vigne, P., Moinier, D., Frelin, C. & Lazdunski, M. A new member of the natriuretic peptide family is present in the venom of the green mamba (Dendroaspis angusticeps). J. Biol. Chem. 267, 13928–13932 (1992).
Sridharan, S., Kini, R. M. & Richards, A. M. Venom natriuretic peptides guide the design of heart failure therapeutics. Pharmacol. Res. 155, 104687 (2020).
Behbehani, M. M. & Pert, A. A mechanism for the analgesic effect of neurotensin as revealed by behavioral and electrophysiological techniques. Brain Res. 324, 35–42 (1984).
Safavi-Hemami, H., Brogan, S. E. & Olivera, B. M. Pain therapeutics from cone snail venoms: from Ziconotide to novel non-opioid pathways. J. Proteomics 190, 12–20 (2019).
Kumar, U. in Peripheral Receptor Targets for Analgesia (ed. Cairns, B. E.) Ch. 16, 397–417 (John Wiley & Sons, 2009).
Ramiro, I. B. L. et al. Somatostatin venom analogs evolved by fish-hunting cone snails: from prey capture behavior to identifying drug leads. Sci. Adv. 8, eabk1410 (2022).
Koch, T. L. et al. Reconstructing the origins of the somatostatin and allatostatin-C signaling systems using the accelerated evolution of biodiverse cone snail venoms. Mol. Biol. Evol. 39, msac075 (2022).
Edgerton, D. S. et al. Targeting insulin to the liver corrects defects in glucose metabolism caused by peripheral insulin delivery. JCI Insight 5, e126974 (2019).
Jensen, M., Hansen, B., De Meyts, P., Schäffer, L. & Ursø, B. Activation of the insulin receptor by insulin and a synthetic peptide leads to divergent metabolic and mitogenic signaling and responses. J. Biol. Chem. 282, 35179–35186 (2007). Important study indicating that ligands with different binding nodes toward IR can lead to biased signaling.
Schäffer, L. et al. A novel high-affinity peptide antagonist to the insulin receptor. Biochem. Biophys. Res. Commun. 376, 380–383 (2008).
De Leon, D. D. & Stanley, C. A. Congenital hypoglycemia disorders: new aspects of etiology, diagnosis, treatment and outcomes: highlights of the Proceedings of the Congenital Hypoglycemia Disorders Symposium, Philadelphia April 2016. Pediatr. Diabetes 18, 3–9 (2017).
Strodel, R. J. & Greene, J. A. Origins of the insulin crisis: how a century of price-fixing controversies affects the cost of care today. Lancet 398, 1793–1795 (2021). Important review on the history of the ‘insulin crisis’.
Blanchette, J. E. et al. GoFundMe as a medical plan: ecological study of crowdfunding insulin success. JMIR Diabetes 7, e33205 (2022).
Beran, D., Ewen, M. & Laing, R. Constraints and challenges in access to insulin: a global perspective. Lancet Diabetes Endocrinol. 4, 275–285 (2016).
Herkert, D. et al. Cost-related insulin underuse among patients with diabetes. JAMA Intern. Med. 179, 112–114 (2019).
Luo, J., Kesselheim, A. S., Greene, J. & Lipska, K. J. Strategies to improve the affordability of insulin in the USA. Lancet 5, 158–159 (2017).
Sachkova, M. Y. et al. Toxin-like neuropeptides in the sea anemone Nematostella unravel recruitment from the nervous system to venom. Proc. Natl Acad. Sci. USA 117, 27481–27492 (2020).
Roelants, K. et al. Origin and functional diversification of an amphibian defense peptide arsenal. PLoS Genet. 9, e1003662 (2013).
Undheim, E. A. et al. Weaponization of a hormone: convergent recruitment of hyperglycemic hormone into the venom of arthropod predators. Structure 23, 1283–1292 (2015).
Eagles, D. A. et al. A peptide toxin in ant venom mimics vertebrate EGF-like hormones to cause long-lasting hypersensitivity in mammals. Proc. Natl Acad. Sci. USA 119, e2112630119 (2022).
LeRoith, D., Shiloach, J., Roth, J. & Lesniak, M. A. Insulin or a closely related molecule is native to Escherichia coli. J. Biol. Chem. 256, 6533–6536 (1981).
Irwin, D. M. Viral hormones: do they impact human endocrinology? Endocrinology 160, 2326–2327 (2019).
Floyd, P. D. et al. Insulin prohormone processing, distribution, and relation to metabolism in Aplysia californica. J. Neurosci. 19, 7732–7741 (1999).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Mirdita, M. et al. ColabFold: making protein folding accessible to all. Nat. Methods 19, 679–682 (2022).
Acknowledgements
We thank T. Lund Koch for assistance with structural predictions of VILPs and sequence analysis of venom insulins, P. Flórez Salcedo for illustrations used in the graphical abstract and Y.W. Zhang for helpful discussion of viral insulin sequences. H.S.-H. and S.H.L. receive support from the Carlsberg Foundation (CF19-0445). D.H.-C.C. receives support from National Institute of Health (DK120430).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
D.H.-C.C. and H.S.-H. hold patents on the insulin analogs mini-Ins G1 and Vh-Ins-HSLQ.
Peer review
Peer review information
Nature Chemical Biology thanks Emrah Altindis and Alexander Zaykov for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Laugesen, S.H., Chou, D.HC. & Safavi-Hemami, H. Unconventional insulins from predators and pathogens. Nat Chem Biol 18, 688–697 (2022). https://doi.org/10.1038/s41589-022-01068-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-022-01068-6