Abstract
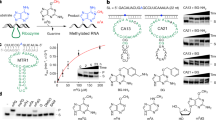
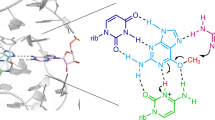
RNA-catalyzed RNA methylation was recently shown to be part of the catalytic repertoire of ribozymes. The methyltransferase ribozyme MTR1 catalyzes the site-specific synthesis of 1-methyladenosine (m1A) in RNA, using O6-methylguanine (m6G) as a methyl group donor. Here, we report the crystal structure of MTR1 at a resolution of 2.8 Å, which reveals a guanine-binding site reminiscent of natural guanine riboswitches. The structure represents the postcatalytic state of a split ribozyme in complex with the m1A-containing RNA product and the demethylated cofactor guanine. The structural data suggest the mechanistic involvement of a protonated cytidine in the methyl transfer reaction. A synergistic effect of two 2′-O-methylated ribose residues in the active site results in accelerated methyl group transfer. Supported by these results, it seems plausible that modified nucleotides may have enhanced early RNA catalysis and that metabolite-binding riboswitches may resemble inactivated ribozymes that have lost their catalytic activity during evolution.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Structural data obtained by X-ray crystallography were deposited in the PDB and are available with the following accession codes: 7Q7X, 7Q7Y, 7Q7Z, 7Q80, 7Q81 and 7Q82. All relevant data are provided in the figures, Extended Data Figs. 1–6, Supplementary Tables 1 and 2 and Supplementary Fig. 1. Publicly available datasets from rcsb.org used in this study include 7OAX, 3SKI, 1Y27, 3FO6, 3MUM, 3Q50 and 7ELR. Source data are provided with this paper.
References
Panchapakesan, S. S. S. & Breaker, R. R. The case of the missing allosteric ribozymes. Nat. Chem. Biol. 17, 375–382 (2021).
Micura, R. & Höbartner, C. Fundamental studies of functional nucleic acids: aptamers, riboswitches, ribozymes and DNAzymes. Chem. Soc. Rev. 49, 7331–7353 (2020).
Kirschning, A. Coenzymes and their role in the evolution of life. Angew. Chem. Int. Ed. 60, 6242–6269 (2021).
Wilson, T. J. & Lilley, D. M. J. The potential versatility of RNA catalysis. Wiley Interdiscip. Rev. RNA 12, e1651 (2021).
Chen, X., Li, N. & Ellington, A. D. Ribozyme catalysis of metabolism in the RNA world. Chem. Biodivers. 4, 633–655 (2007).
Breaker, R. R. Imaginary ribozymes. ACS Chem. Biol. 15, 2020–2030 (2020).
Jadhav, V. R. & Yarus, M. Coenzymes as coribozymes. Biochimie 84, 877–888 (2002).
Winkler, W. C., Nahvi, A., Roth, A., Collins, J. A. & Breaker, R. R. Control of gene expression by a natural metabolite-responsive ribozyme. Nature 428, 281–286 (2004).
Silverman, S. K. Catalytic DNA: scope, applications, and biochemistry of deoxyribozymes. Trends Biochem. Sci. 41, 595–609 (2016).
Cernak, P. & Sen, D. A thiamin-utilizing ribozyme decarboxylates a pyruvate-like substrate. Nat. Chem. 5, 971–977 (2013).
Tsukiji, S., Pattnaik, S. B. & Suga, H. An alcohol dehydrogenase ribozyme. Nat. Struct. Mol. Biol. 10, 713–717 (2003).
Ishida, S., Terasaka, N., Katoh, T. & Suga, H. An aminoacylation ribozyme evolved from a natural tRNA-sensing T-box riboswitch. Nat. Chem. Biol. 16, 702–709 (2020).
Jadhav, V. R. & Yarus, M. Acyl-CoAs from coenzyme ribozymes. Biochemistry 41, 723–729 (2002).
Serganov, A. et al. Structural basis for Diels–Alder ribozyme-catalyzed carbon–carbon bond formation. Nat. Struct. Mol. Biol. 12, 218–224 (2005).
Robertson, M. P. & Scott, W. G. The structural basis of ribozyme-catalyzed RNA assembly. Science 315, 1549–1553 (2007).
Shechner, D. M. et al. Crystal structure of the catalytic core of an RNA-polymerase ribozyme. Science 326, 1271–1275 (2009).
Ponce-Salvatierra, A., Wawrzyniak-Turek, K., Steuerwald, U., Höbartner, C. & Pena, V. Crystal structure of a DNA catalyst. Nature 529, 231–234 (2016).
Scheitl, C. P. M., Ghaem Maghami, M., Lenz, A. K. & Höbartner, C. Site-specific RNA methylation by a methyltransferase ribozyme. Nature 587, 663–667 (2020).
Flemmich, L., Heel, S., Moreno, S., Breuker, K. & Micura, R. A natural riboswitch scaffold with self-methylation activity. Nat. Commun. 12, 3877 (2021).
Serganov, A. et al. Structural basis for discriminative regulation of gene expression by adenine- and guanine-sensing mRNAs. Chem. Biol. 11, 1729–1741 (2004).
Gilbert, S. D., Reyes, F. E., Edwards, A. L. & Batey, R. T. Adaptive ligand binding by the purine riboswitch in the recognition of guanine and adenine analogs. Structure 17, 857–868 (2009).
Wolk, S. K. et al. Modified nucleotides may have enhanced early RNA catalysis. Proc. Natl Acad. Sci. USA 117, 8236–8242 (2020).
Robertson, M. P. & Scott, W. G. A general method for phasing novel complex RNA crystal structures without heavy-atom derivatives. Acta Crystallogr. D Biol Crystallogr. D64, 738–744 (2008).
Höbartner, C. & Micura, R. Chemical synthesis of selenium-modified oligoribonucleotides and their enzymatic ligation leading to an U6SnRNA stem-loop segment. J. Am. Chem. Soc. 126, 1141–1149 (2004).
Regulski, E. E. & Breaker, R. R. In-line probing analysis of riboswitches. Meth. Mol. Biol. 419, 53–67 (2008).
Gaffney, B. L., Goswami, B. & Jones, R. A. Nitrogen-15-labeled oligodeoxynucleotides. 7. Use of nitrogen-15 NMR to probe hydrogen bonding in an O6MeG:C base pair. J. Am. Chem. Soc. 115, 12607–12608 (1993).
Batey, R. T., Gilbert, S. D. & Montange, R. K. Structure of a natural guanine-responsive riboswitch complexed with the metabolite hypoxanthine. Nature 432, 411–415 (2004).
Pikovskaya, O., Polonskaia, A., Patel, D. J. & Serganov, A. Structural principles of nucleoside selectivity in a 2′-deoxyguanosine riboswitch. Nat. Chem. Biol. 7, 748–755 (2011).
Keller, H., Weickhmann, A. K., Bock, T. & Wohnert, J. Adenine protonation enables cyclic-di-GMP binding to cyclic-GAMP sensing riboswitches. RNA 24, 1390–1402 (2018).
Wolter, A. C. et al. A stably protonated adenine nucleotide with a highly shifted pKa value stabilizes the tertiary structure of a GTP-binding RNA aptamer. Angew. Chem. Int. Ed. 56, 401–404 (2017).
Freire, F. et al. A simple NMR analysis of the protonation equilibrium that accompanies aminoglycoside recognition: dramatic alterations in the neomycin-B protonation state upon binding to a 23-mer RNA aptamer. Chem. Commun. 43, 174–176 (2007).
Mandal, M., Boese, B., Barrick, J. E., Winkler, W. C. & Breaker, R. R. Riboswitches control fundamental biochemical pathways in Bacillus subtilis and other bacteria. Cell 113, 577–586 (2003).
Noeske, J. et al. An intermolecular base triple as the basis of ligand specificity and affinity in the guanine- and adenine-sensing riboswitch RNAs. Proc. Natl Acad. Sci. USA 102, 1372–1377 (2005).
Wilcox, J. L., Ahluwalia, A. K. & Bevilacqua, P. C. Charged nucleobases and their potential for RNA catalysis. Acc. Chem. Res. 44, 1270–1279 (2011).
Krishnamurthy, R. Role of pKa of nucleobases in the origins of chemical evolution. Acc. Chem. Res. 45, 2035–2044 (2012).
Abou Assi, H. et al. 2′-O-Methylation can increase the abundance and lifetime of alternative RNA conformational states. Nucl. Acids Res. 48, 12365–12379 (2020).
Chatterjee, S. et al. The chemical nature of the 2′-substituent in the pentose-sugar dictates the pseudoaromatic character of the nucleobase (pKa) in DNA/RNA. Org. Biomol. Chem. 4, 1675–1686 (2006).
Olsen, D. B., Benseler, F., Aurup, H., Pieken, W. A. & Eckstein, F. Study of a hammerhead ribozyme containing 2′-modified adenosine residues. Biochemistry 30, 9735–9741 (1991).
Schubert, S. et al. RNA cleaving ‘10–23’ DNAzymes with enhanced stability and activity. Nucl. Acids Res. 31, 5982–5992 (2003).
Wang, Y., Nguyen, K., Spitale, R. C. & Chaput, J. C. A biologically stable DNAzyme that efficiently silences gene expression in cells. Nat. Chem. 13, 319–326 (2021).
Xu, X. et al. Insights into xanthine riboswitch structure and metal ion-mediated ligand recognition. Nucl. Acids Res. 49, 7139–7153 (2021).
Ghaem Maghami, M., Scheitl, C. P. M. & Höbartner, C. Direct in vitro selection of trans-acting ribozymes for posttranscriptional, site-specific, and covalent fluorescent labeling of RNA. J. Am. Chem. Soc. 141, 19546–19549 (2019).
Höbartner, C. et al. The synthesis of 2′-O-[(triisopropylsilyl)oxy] methyl (TOM) phosphoramidites of methylated ribonucleosides (m1G, m2G, m22G, m1I, m3U, m4C, m6A, m62A) for use in automated RNA solid-phase synthesis. Monatsh. Chem. 134, 851–873 (2003).
Moroder, H., Kreutz, C., Lang, K., Serganov, A. & Micura, R. Synthesis, oxidation behavior, crystallization and structure of 2′-methylseleno guanosine containing RNAs. J. Am. Chem. Soc. 128, 9909–9918 (2006).
Kabsch, W. Xds. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Evans, P. R. An introduction to data reduction: space-group determination, scaling and intensity statistics. Acta Crystallogr. D Biol. Crystallogr. 67, 282–292 (2011).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Mieczkowski, M. et al. Large Stokes shift fluorescence activation in an RNA aptamer by intermolecular proton transfer to guanine. Nat. Commun. 12, 3549 (2021).
Terwilliger, T. C. et al. Iterative model building, structure refinement and density modification with the PHENIX AutoBuild wizard. Acta Crystallogr. D Biol. Crystallogr. 64, 61–69 (2008).
Emsley, P. & Cowtan, K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004).
Afonine, P. V. et al. Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr. D Biol. Crystallogr. 68, 352–367 (2012).
Lebedev, A. A. et al. JLigand: a graphical tool for the CCP4 template-restraint library. Acta Crystallogr. D Biol. Crystallogr. 68, 431–440 (2012).
Moriarty, N. W., Grosse-Kunstleve, R. W. & Adams, P. D. electronic Ligand Builder and Optimization Workbench (eLBOW): a tool for ligand coordinate and restraint generation. Acta Crystallogr. D Biol. Crystallogr. 65, 1074–1080 (2009).
Acknowledgements
This work was supported by the European Research Council (ERC; 682586), the Deutsche Forschungsgemeinschaft (DFG; HO4436/3-1) and the University of Würzburg. We thank C. Pfeuffer and A. Lenz for technical assistance and the beamline staff at DESY (PETRA III, P11) and ESRF (ID23) for assistance with data collection.
Author information
Authors and Affiliations
Contributions
C.P.M.S. and C.H. designed the study. H.S. and C.P.M.S. collected diffraction data. M.M. solved the structure. C.P.M.S. performed biochemical experiments. C.H. wrote the paper with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
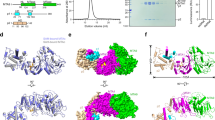
Extended Data Fig. 1 Heavy atom derivatives of MTR1 crystal structure.
(a) Overall structure of MTR1 co-crystallized with Tl+. (b) MTR1 containing 2′-Selenomethyl-uridine modified residue in the RNA substrate. Yellow mesh indicates anomalous difference Fourier map contoured at (a) 3σ and (b) 5σ. The difference maps were computed from data collected at the Thallium L-III edge and Selenium K-edge, respectively.
Extended Data Fig. 2 Overall structures of MTR1 and crystal contacts.
(a) Secondary structure scheme of trimolecular MTR1. (b) Packing of overhangs of P1 and P2 to form a semi-continuous double helix. Arrangement of two copies in the asymmetric unit for crystals grown with (c) ab6G (ab1A in the product) and (d) with m6G (m1A in the crystal) but no added Mg2+. (e) Crystal contact via stacking of P3 in the structure shown in (d). (f,g) Anion exchange HPLC analyses of dissolved crystals corresponding to (c) and (d).
Extended Data Fig. 3 In-line probing of MTR1.
(a-c) Full gel images for the excerpts shown in Fig. 2. (a) In-line probing of MTR1 hybridized to unmethylated RNA R1, with increasing concentrations of m6G. (b) In-line probing of guanine binding to the MTR1 product complex containing m1A. (c) In-line probing of guanine binding to the MTR1 starting complex hybridized to unmethylated RNA R1. (a, b, c) Incubation at pH 8.0, 20 °C, 36 h. (d) Normalized band intensities seen in (a), shown for U36 (red), A37 (black), G38 (blue). The [m6G]1/2 value is ca 800 µM, however, this value cannot be interpreted as a Kd or Km because of multiple overlapping equilibria (with m6G and G since partial/slow methylation occurred during incubation). (e) Normalized in-line probing band intensities seen in (b) for U36 (red), A37 (black), G38 (blue). Kd,app = 2.0 ± 0.3 µM. Data in d) and e) are fitted to a one-site binding model. Error bars denote ± s.d. of the mean for n = 3 (d) or 4 (e) independent replicates. (f) Secondary structure scheme of MTR1 used in in-line probing experiments (with connecting loop at P3; numbers of nucleotides correspond to the split version). (g) Excerpt of the catalytic core showing solvent exposed location of U36 and stacking of A37 on m1A. (h) Excerpt of the metal ion binding site in the transition of the catalytic core to P3.
Extended Data Fig. 4 MTR1 mutagenesis of the core nucleotides and pH dependence.
(a) Scheme of MTR1 with mutated nucleotides and base-pairs indicated. (b) Summary of rate constants determined at pH 6.0 and 7.5. Individual data points of two independent replicates are shown as black dots. Reaction of MTR1-wt at pH 7.5 war repeated three times. (c) Representative gel images of kinetic experiments of two independent replicates. Reaction conditions: 5′-32P-labeled R1, 10 µM MTR1 or mutant, 100 µM m6G, 40 mM MgCl2, 25 °C. Timepoints: 0, 0.2, 0.5, 1, 2, 4, 7, 23 h (ON).
Extended Data Fig. 5 MTR1 as alkyltransferase ribozyme and pH dependence.
(a, b) Chemical structures of substrates (adenosine and benzyl guanine cofactor) before the RNA-catalyzed reaction, and after transfer to the target adenosine in R1 and release of guanine. (c) Overlay of active sites containing m1A, bn1A or ab1A in stick representation. (d) Kinetics of benzyl group transfer at pH 6.0 and pH 7.5, with exemplary gel images as inset. (e) Same as (d), but reaction with ab6G. The fastest reaction was observed with ab6G at pH 6.0, yielding ca 90% ab1A-RNA within 2 min. All reactions were performed in duplicate, individual data points and representative gel images are shown (timepoints shown in gels up to 60 min).
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2 and Fig. 1.
Source data
Source Data Fig. 2
Statistical source data.
Source Data Fig. 3
Individual data points.
Source Data Fig. 3
Unprocessed gels.
Source Data Fig. 4
Individual data points.
Source Data Fig. 4
Unprocessed gels.
Source Data Extended Data Fig. 3
Statistical source data.
Source Data Extended Data Fig. 4
Individual data points.
Source Data Extended Data Fig. 4
Unprocessed gels.
Source Data Extended Data Fig. 5
Individual data points.
Source Data Extended Data Fig. 5
Unprocessed gels.
Rights and permissions
About this article
Cite this article
Scheitl, C.P.M., Mieczkowski, M., Schindelin, H. et al. Structure and mechanism of the methyltransferase ribozyme MTR1. Nat Chem Biol 18, 547–555 (2022). https://doi.org/10.1038/s41589-022-00976-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-022-00976-x
This article is cited by
-
A SAM analogue-utilizing ribozyme for site-specific RNA alkylation in living cells
Nature Chemistry (2023)
-
A new RNA performs old chemistry
Nature Chemical Biology (2022)
-
An RNA aptamer that shifts the reduction potential of metabolic cofactors
Nature Chemical Biology (2022)