Abstract
Disaggregation of amyloid fibrils is a fundamental biological process required for amyloid propagation. However, due to the lack of experimental systems, the molecular mechanism of how amyloid is disaggregated by cellular factors remains poorly understood. Here, we established a robust in vitro reconstituted system of yeast prion propagation and found that heat-shock protein 104 (Hsp104), Ssa1 and Sis1 chaperones are essential for efficient disaggregation of Sup35 amyloid. Real-time imaging of single-molecule fluorescence coupled with the reconstitution system revealed that amyloid disaggregation is achieved by ordered, timely binding of the chaperones to amyloid. Remarkably, we uncovered two distinct prion strain conformation-dependent modes of disaggregation, fragmentation and dissolution. We characterized distinct chaperone dynamics in each mode and found that transient, repeated binding of Hsp104 to the same site of amyloid results in fragmentation. These findings provide a physical foundation for otherwise puzzling in vivo observations and for therapeutic development for amyloid-associated neurodegenerative diseases.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Additional data are available from the corresponding author upon reasonable request. Source data are provided with this paper.
References
Eisenberg, D. & Jucker, M. The amyloid state of proteins in human diseases. Cell 148, 1188–1203 (2012).
Shorter, J. Designer protein disaggregases to counter neurodegenerative disease. Curr. Opin. Genet. Dev. 44, 1–8 (2017).
Vaquer-Alicea, J. & Diamond, M. I. Propagation of protein aggregation in neurodegenerative diseases. Annu. Rev. Biochem. 88, 785–810 (2019).
Marrero-Winkens, C., Sankaran, C. & Schätzl, H. M. From seeds to fibrils and back: fragmentation as an overlooked step in the propagation of prions and prion-like proteins. Biomolecules 10, 1305 (2020).
Tanaka, M. & Komi, Y. Layers of structure and function in protein aggregation. Nat. Chem. Biol. 11, 373–377 (2015).
Scheckel, C. & Aguzzi, A. Prions, prionoids and protein misfolding disorders. Nat. Rev. Genet. 19, 405–418 (2018).
Tanaka, M., Collins, S. R., Toyama, B. H. & Weissman, J. S. The physical basis of how prion conformations determine strain phenotypes. Nature 442, 585–589 (2006).
Doyle, S. M. & Wickner, S. Hsp104 and ClpB: protein disaggregating machines. Trends Biochem. Sci. 34, 40–48 (2009).
Mogk, A., Kummer, E. & Bukau, B. Cooperation of Hsp70 and Hsp100 chaperone machines in protein disaggregation. Front. Mol. Biosci. 2, 22 (2015).
Liebman, S. W. & Chernoff, Y. O. Prions in yeast. Genetics 191, 1041–1072 (2012).
Wickner, R. B. et al. Yeast prions: structure, biology, and prion-handling systems. Microbiol. Mol. Biol. Rev. 79, 1–17 (2015).
Tuite, M. F. & Serio, T. R. The prion hypothesis: from biological anomaly to basic regulatory mechanism. Nat. Rev. Mol. Cell Biol. 11, 823–833 (2010).
Chernoff, Y. O., Lindquist, S. L., Ono, B. I., Inge-Vechtomov, S. G. & Liebman, S. W. Role of the chaperone protein Hsp104 in propagation of the yeast prion-like factor [psi+]. Science 268, 880–884 (1995).
Shorter, J. & Lindquist, S. Hsp104 catalyzes formation and elimination of self-replicating Sup35 prion conformers. Science 304, 1793–1797 (2004).
Glover, J. R. et al. Self-seeded fibers formed by Sup35, the protein determinant of [PSI+], a heritable prion-like factor of S. cerevisiae. Cell 89, 811–819 (1997).
Desantis, M. E. et al. Operational plasticity enables Hsp104 to disaggregate diverse amyloid and nonamyloid clients. Cell 151, 778–793 (2012).
Desantis, M. E. & Shorter, J. Hsp104 drives ‘protein-only’ positive selection of sup35 prion strains encoding strong [PSI+]. Chem. Biol. 19, 1400–1410 (2012).
Inoue, Y., Taguchi, H., Kishimoto, A. & Yoshida, M. Hsp104 binds to yeast Sup35 prion fiber but needs other factor(s) to sever it. J. Biol. Chem. 279, 52319–52323 (2004).
Johnston, D. M., Miot, M., Hoskins, J. R., Wickner, S. & Doyle, S. M. Substrate discrimination by ClpB and Hsp104. Front. Mol. Biosci. 4, 36 (2017).
Jung, G., Jones, G., Wegrzyn, R. D. & Masison, D. C. A role for cytosolic Hsp70 in yeast [PSI+] prion propagation and [PSI+] as a cellular stress. Genetics 156, 559–570 (2000).
Jones, G. W. & Masison, D. C. Saccharomyces cerevisiae Hsp70 mutations affect [PSI+] prion propagation and cell growth differently and implicate Hsp40 and tetratricopeptide repeat cochaperones in impairment of [PSI+]. Genetics 163, 495–506 (2003).
Higurashi, T., Hines, J. K., Sahi, C., Aron, R. & Craig, E. A. Specificity of the J-protein Sis1 in the propagation of 3 yeast prions. Proc. Natl Acad. Sci. USA 105, 16596–16601 (2008).
Tipton, K. A., Verges, K. J. & Weissman, J. S. In vivo monitoring of the prion replication cycle reveals a critical role for Sis1 in delivering substrates to Hsp104. Mol. Cell 32, 584–591 (2008).
Winkler, J., Tyedmers, J., Bukau, B. & Mogk, A. Hsp70 targets Hsp100 chaperones to substrates for protein disaggregation and prion fragmentation. J. Cell Biol. 198, 387–404 (2012).
Newnam, G. P., Wegrzyn, R. D., Lindquist, S. L. & Chernoff, Y. O. Antagonistic interactions between yeast chaperones Hsp104 and Hsp70 in prion curing. Mol. Cell. Biol. 19, 1325–1333 (1999).
Parsell, D. A., Kowal, A. S. & Lindquist, S. Saccharomyces cerevisiae Hsp104 protein. Purification and characterization of ATP-induced structural changes. J. Biol. Chem. 269, 4480–4487 (1994).
Parsell, D. A., Kowal, A. S., Singer, M. A. & Lindquist, S. Protein disaggregation mediated by heat-shock protein Hsp104. Nature 372, 475–478 (1994).
Krzewska, J. & Melki, R. Molecular chaperones and the assembly of the prion Sup35p, an in vitro study. EMBO J. 25, 822–833 (2006).
Shorter, J. & Lindquist, S. Hsp104, Hsp70 and Hsp40 interplay regulates formation, growth and elimination of Sup35 prions. EMBO J. 27, 2712–2724 (2008).
Uchiyama, S., Noda, M. & Krayukhina, E. Sedimentation velocity analytical ultracentrifugation for characterization of therapeutic antibodies. Biophys. Rev. 10, 259–269 (2018).
Ohhashi, Y., Ito, K., Toyama, B. H., Weissman, J. S. & Tanaka, M. Differences in prion strain conformations result from non-native interactions in a nucleus. Nat. Chem. Biol. 6, 225–230 (2010).
Tanaka, M., Chien, P., Naber, N., Cooke, R. & Weissman, J. S. Conformational variations in an infectious protein determine prion strain differences. Nature 428, 323–328 (2004).
Toyama, B. H., Kelly, M. J. S., Gross, J. D. & Weissman, J. S. The structural basis of yeast prion strain variants. Nature 449, 233–237 (2007).
Ohhashi, Y. et al. Molecular basis for diversification of yeast prion strain conformation. Proc. Natl Acad. Sci. USA 115, 2389–2394 (2018).
Kryndushkin, D. S., Alexandrov, I. M., Ter-Avanesyan, M. D. & Kushnirov, V. V. Yeast [PSI+] prion aggregates are formed by small Sup35 polymers fragmented by Hsp104. J. Biol. Chem. 278, 49636–49643 (2003).
Bösl, B., Grimminger, V. & Walter, S. Substrate binding to the molecular chaperone Hsp104 and its regulation by nucleotides. J. Biol. Chem. 280, 38170–38176 (2005).
Tsai, J. & Douglas, M. G. A conserved HPD sequence of the J-domain is necessary for YDJ1 stimulation of Hsp70 ATPase activity at a site distinct from substrate binding. J. Biol. Chem. 271, 9347–9354 (1996).
Yan, W. & Craig, E. A. The glycine–phenylalanine-rich region determines the specificity of the yeast Hsp40 Sis1. Mol. Cell. Biol. 19, 7751–7758 (1999).
Rosenzweig, R., Moradi, S., Zarrine-Afsar, A., Glover, J. R. & Kay, L. E. Unraveling the mechanism of protein disaggregation through a ClpB–DnaK interaction. Science 339, 1080–1083 (2013).
Haslberger, T. et al. M domains couple the ClpB threading motor with the DnaK chaperone activity. Mol. Cell 25, 247–260 (2007).
Lum, R., Niggemann, M. & Glover, J. R. Peptide and protein binding in the axial channel of Hsp104: insights into the mechanism of protein unfolding. J. Biol. Chem. 283, 30139–30150 (2008).
Avellaneda, M. J. et al. Processive extrusion of polypeptide loops by a Hsp100 disaggregase. Nature 578, 317–320 (2020).
De Los Rios, P., Ben-Zvi, A., Slutsky, O., Azem, A. & Goloubinoff, P. Hsp70 chaperones accelerate protein translocation and the unfolding of stable protein aggregates by entropic pulling. Proc. Natl Acad. Sci. USA 103, 6166–6171 (2006).
Gao, X. et al. Human Hsp70 disaggregase reverses Parkinson’s-linked α-synuclein amyloid fibrils. Mol. Cell 59, 781–793 (2015).
Allen, K. D. et al. Hsp70 chaperones as modulators of prion life cycle: novel effects of Ssa and Ssb on the Saccharomyces cerevisiae prion [PSI+]. Genetics 169, 1227–1242 (2005).
Mayer, M. P. Hsp70 chaperone dynamics and molecular mechanism. Trends Biochem. Sci. 38, 507–514 (2013).
Rosenzweig, R., Nillegoda, N. B., Mayer, M. P. & Bukau, B. The Hsp70 chaperone network. Nat. Rev. Mol. Cell Biol. 20, 665–680 (2019).
Frederick, K. K. et al. Distinct prion strains are defined by amyloid core structure and chaperone binding site dynamics. Chem. Biol. 21, 295–305 (2014).
Bagriantsev, S. N., Gracheva, E. O., Richmond, J. E. & Liebman, S. W. Variant-specific [PSI+] infection is transmitted by Sup35 polymers within [PSI+] aggregates with heterogeneous protein composition. Mol. Biol. Cell 19, 2433–2443 (2008).
Wentink, A. S. et al. Molecular dissection of amyloid disaggregation by human HSP70. Nature 587, 483–488 (2020).
Sweeny, E. A., Desantis, M. E. & Shorter, J. Purification of Hsp104, a protein disaggregase. J. Vis. Exp. https://doi.org/10.3791/3190 (2011).
Endo, T., Mitsui, S., Nakai, M. & Roise, D. Binding of mitochondrial presequences to yeast cytosolic heat shock protein 70 depends on the amphiphilicity of the presequence. J. Biol. Chem. 271, 4161–4167 (1996).
Hanashima, S. et al. Defining the interaction of human soluble lectin ZG16p and mycobacterial phosphatidylinositol mannosides. ChemBioChem 16, 1502–1511 (2015).
Schuck, P. Size-distribution analysis of macromolecules by sedimentation velocity ultracentrifugation and Lamm equation modeling. Biophys. J. 78, 1606–1619 (2000).
Jackrel, M. E. et al. Potentiated Hsp104 variants antagonize diverse proteotoxic misfolding events. Cell 156, 170–182 (2014).
Shorter, J. The mammalian disaggregase machinery: Hsp110 synergizes with Hsp70 and Hsp40 to catalyze protein disaggregation and reactivation in a cell-free system. PLoS ONE 6, e26319 (2011).
Glover, J. R. & Lindquist, S. Hsp104, Hsp70, and Hsp40. Cell 94, 73–82 (1998).
Bagriantsev, S. & Liebman, S. W. Specificity of prion assembly in vivo: [PSI+] and [PIN+] form separate structures in yeast. J. Biol. Chem. 279, 51042–51048 (2004).
Okamoto, K. & Sako, Y. Single-molecule Förster resonance energy transfer measurement reveals the dynamic partially ordered structure of the epidermal growth factor receptor C-tail domain. J. Phys. Chem. B 123, 571–581 (2019).
Tseng, Q. et al. A new micropatterning method of soft substrates reveals that different tumorigenic signals can promote or reduce cell contraction levels. Lab Chip 11, 2231–2240 (2011).
Acknowledgements
We thank N. Takahashi and E. Uemura for reagent and protein preparation, H. Martadinata, T. Nomura, H. Kurahashi, H. Kimura and J. Burke for providing advice on experiments and comments on the manuscript and members of the Tanaka laboratory for discussions. DNA sequencing was performed by the RIKEN Center for Brain Science, Research Resources Division. We are grateful to the Support Unit for Bio-Material Analysis, RIKEN CBS Research Resources Division. This work was supported, in part, by the Grants-in-Aid for Scientific Research (A) (20H00501 to M.T.), for Innovative Areas (20H04720 to M.T.; 26116002 to H.T.) and for Transformative Research Area (A) (21H05257 to M.T.) from the Ministry of Education, Culture, Sports, Science and Technology, Japan, the RIKEN Aging project (to M.T. and M.S.), the RIKEN Pioneering Project (Biology of Intracellular Environments) (to M.T.), Takeda Science Foundation (Visionary Research) (to M.T.), Japan Foundation for Applied Enzymology (to M.T.), and Nakatani Foundation (to M.T.). Y.N. is a recipient of the Junior Research Associate fellowship from RIKEN.
Author information
Authors and Affiliations
Contributions
Y.N. and M.T. conceived and designed the whole study. Y.N. prepared all proteins except for Ydj1 and performed all experiments except for sedimentation velocity AUC experiments using Rayleigh interferometry and a fluorescence detection system and negative staining electron microscopy experiments. Y.N., Y.T., T.Y. and M.S. performed negative staining experiments. T.K., E.K. and S.U. performed the sedimentation velocity AUC experiments using Rayleigh interferometry and a fluorescence detection system and analyzed the data. H.C.-H.S., Y.K. and S.S. prepared some of the plasmids and recombinant proteins. K.O., M.I., T.N., Y.S. and H.T. provided suggestions for TIRF imaging experiments and data analysis. Y.N. and M.T. wrote the manuscript, and all authors provided comments. M.T. supervised the whole project.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Chemical Biology thanks Tommer Ravid, Sander Tans and other anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
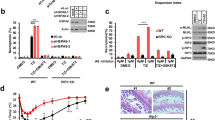
Extended Data Fig. 1 Hsp104, Ssa1 and Sis1 chaperones have a Sup35NM remodeling activity.
a, Hsp104, Ssa1, Sis1, and Ydj1 proteins purified in this study were detected by SDS-PAGE. b, Sedimentation coefficient distribution of Hsp104 at 30 μM was obtained by AUC analysis. c-d, Morphology of Sup35NM amyloid in acceleration of amyloid formation was observed by AFM. (c) Morphology of Sup35NM protein during the acceleration process of amyloid formation (0-45 min). (d) Morphology of Sup35NM amyloid fibrils formed spontaneously or by Hsp104/Ssa1/Sis1 or Hsp104KT/Ssa1/Sis1 after plateau (~ 48 hours). e, 20 μM Sup35NM monomer including 20 nM Alexa488 labeled Sup35NM were incubated with 20 μM Hsp104, 20 μM Ssa1, and 20 μM Sis1 in the presence of ATPγS (left) and ADP (right) at 20oC. Interactions with Sup35NM with chaperone(s) were investigated by AUC with a fluorescence detection system. The inset shows the range of sedimentation coefficient between 13 and 30S.
Extended Data Fig. 2 Sup35NM amyloid is efficiently disaggregated by Hsp104, Ssa1 and Sis1 chaperones.
a, 0.5 μM Sc4 amyloid was incubated with various concentrations (0.01, 0.1, 1, 10, and 30 μM) of Hsp104 in the presence of 2 μM Ssa1 and 2 μM Sis1. Data were averaged from 3 independent experiments (n = 3). Data are presented as mean values +/− S.D. Notably, the reduced disaggregation activity of Hsp104 under its high concentration is likely caused by the inhibition of Ssa1/Sis binding to the fibrils due to the enhanced accumulation of Hsp104 on fibrils25. b, 0.5 μM Sc4 amyloid was incubated with various concentrations (0.1, 0.5, 1, 2, and 4 μM) of Ssa1 in the presence of 1 μM Hsp104 and 2 μM Sis1. Data were averaged from 2 independent experiments (n = 2). c, 0.5 μM Sc4 amyloid was incubated with various concentrations (0.1, 0.5, 1, 2, and 4 μM) of Sis1 in the presence of 1 μM Hsp104 and 2 μM Ssa1. Data were averaged from 2 independent experiments (n = 2). d, 0.5 μM Sc4 amyloid was incubated with 1 μM Hsp104 or Hsp104 KT with 2 μM Ssa1, 2 μM Sis1 for 2 hours at 30oC. Soluble Sup35NM protein that was disaggregated from Sc4 amyloid was separated by ultracentrifugation. e, Quantification of signal intensity of soluble (S) and total amyloid-derived (T) Sup35NM protein in Extended Data Fig. 2d. Relative soluble fraction (S/T) was calculated. Data were averaged from three independent experiments (n = 3). Data are presented as mean values +/− S.D. Statistical significance was analyzed by unpaired one-way ANOVA. ** P < 0.01. f, 50 nM urea-denatured amorphous luciferase aggregates were incubated with 1 μM Hsp104, 2 μM Ssa1, 1 μM Sis1 or Ydj1, and luminescence of luciferase was monitored for 240 min at 30oC. Data were normalized by the value of native luciferase control. Data were averaged from 2 independent experiments (n = 2). g, 0.5 μM Sc4 amyloid was incubated with 1 μM Hsp104, 2 μM Ssa1, 2 μM Sis1 or Ydj1, and thioflavin T fluorescence was monitored at 30oC. Data were normalized by the initial fluorescence intensities and averaged from independent experiments (n = 7 for Sc4, n = 9 for Sc4 + Hsp104/Ssa1/Sis1, Sc4 + Hsp104/Ssa1/Ydj1, Sc4 + Hsp104/Ssa1). Data are presented as mean values +/− S.D.
Extended Data Fig. 3 Activities and interactions of Hsp104-SNAP549 or Hsp104trap-SNAP549 with Sc4 amyloid were analyzed by biophysical methods.
a, Schematic representation of the single molecule imaging of Sup35NM amyloid disaggregation. STELLA650 labeled Sup35NM amyloid (Sc4 or Sc37 amyloid) was immobilized on flow-cells with PEG coating and neutravidin treatment. Fluorescently labeled chaperones were introduced into the flow-cell, and fluorescence signal intensities were detected when chaperone(s) interacted with Sup35NM amyloid. b, Representative time-lapse TIRF images from Supplementary Video 2 showing interactions between Sc4 amyloid-STELLA650 and Hsp104-SNAP549. Sc4 amyloid-STELLA650 (red) was observed after various combinations of 40 nM Hsp104-SNAP549 (green), 2 μM Ssa1 and 2 μM Sis1 are mixed and flowed. Scale bar, 3 μm. Images were acquired every 500 msec. c, Disaggregation of Sc4 amyloid was impaired in the presence of Hsp104trap with Ssa1/Sis1. Kinetics of disaggregation of 0.5 μM Sc4 amyloid with various combinations of 1 μM Hsp104 or Hsp104trap, 2 μM Ssa1, 2 μM Sis1 was monitored at 30 °C by thioflavin T fluorescence. Data were normalized by the initial fluorescence intensities. Data were averaged from 2 independent experiments (n = 2). d, Binding affinities of Sc4 amyloid to Hsp104trap in the presence (red) or absence (black) of Ssa1 and Sis1 were measured by microscale thermophoresis. All data were averaged from 3 independent experiments (n = 3). Data are presented as mean values +/− S.D.
Extended Data Fig. 4 Sis1 requires Ssa1 and ATP for its binding to Sc4 amyloid.
a, Representative time-lapse TIRF images from Supplementary Video 3 showing interactions between Sc4 amyloid-STELLA650 and Ssa1-Alexa488. Sc4 amyloid-STELLA650 (red) was observed after various combinations of 2 μM Ssa1-Alexa488 (cyan) and 0.5 μM Sis1 with a distinct status of ATP were mixed and flowed. Images were acquired every 10 sec. Scale bar, 3 μm. b, Representative time-lapse TIRF images showing interactions between Sc4 amyloid-STELLA650 and Sis1-Cy3, after Sc4 amyloid-STELLA650, 0.5 μM Sis1-Cy3 and 2 μM Ssa1 with ATP or ATPγS were mixed and flowed. Images were acquired every 10 sec. Scale bar, 3 μm. c, Kinetics of accumulation of Sis1-Cy3 fluorescence on Sc4 amyloid in the presence or absence of Sis1 with ATP or ATPγS. All data were averaged from 3 independent fibers (n = 3) in 1 observation for each condition. Data are presented as mean values +/− S.D.
Extended Data Fig. 5 Ssa1 and Sis1 form a complex and simultaneously bind to Sc4 amyloid.
a, Sedimentation coefficient distribution of Ssa1, Sis1, Sis1-HPD/AAA, Ssa1+Sis1 and Ssa1+Sis1-HPD/AAA at a concentration of 4 μM including 5 mM of ATP was obtained by AUC in 20oC. Sedimentation was conducted at velocity of 42,000 rpm. Number of scans was 300 (scan interval: 2 min). (top) overall distribution at a range of 0-42S. (bottom, left) Enlarged view at a range of 0.5-8S. (bottom, right) Enlarged view at a range of 8-42S. b, Kinetics of accumulation of Ssa1-Alexa488 fluorescence on Sc4 amyloid in the presence of Sis1-Cy3 or Sis1 HPD/AAA-Cy3 with 5 mM ATP. 8 fibrils were analyzed from 2 observations for each condition. Data are presented as mean values +/− S.D. c, Kinetics of accumulation of Sis1-Cy3 or Sis1 HPD/AAA-Cy3 fluorescence on Sc4 amyloid in the presence of Ssa1-Alexa488 with 5 mM ATP. 8 fibrils were analyzed from 2 observations for each condition. Data are presented as mean values +/− S.D.
Extended Data Fig. 6 Hsp104 binds to both of substrate (amyloid) and Ssa1 bound onto amyloid.
a, Kinetics of disaggregation of 0.5 μM Sc4 amyloid with 1 μM Hsp104 or Hsp104 mutants (Y507A, Y257A), 2 μM Ssa1 and 2 μM Sis1 was monitored at 30 °C by thioflavin T fluorescence. Data were normalized by the initial fluorescence intensities and averaged from 6 independent experiments (n = 6). Data are presented as mean values +/− S.D. b, Representative TIRF images showing disaggregation of Sc4 amyloid-STELLA650 at various time points. Sc4 amyloid-STELLA650 was observed after 1 μM Hsp104 or Hsp104 mutants (Y507A, Y257A), 2 μM Ssa1 and 2 μM Sis1 were mixed and flowed. Scale bar, 5 μm. c, Analysis of fragmentation rate of Sc4 amyloid in b. Each data point shows the cumulative number of fragmentation events (per μm amyloid) at various time points. Data were averaged from 5 independent fibers (n = 5 from 1 observation). Data are presented as mean values +/− S.D. d, Representative time-lapse TIRF images showing interactions between Sc4 amyloid-STELLA650, Hsp104 WT, Y507A or Y257A-SNAP549, after 10 nM Hsp104-SNAP549, 2 μM Ssa1 and 0.5 μM Sis1 were mixed and flowed. Images were acquired every 500 msec. Scale bar, 5 μm. e, Number of Hsp104 WT or mutants binding (appearance) on Sc4 amyloid during 150 sec. The number of events was averaged from n = 8 fibrils for Hsp104WT, n = 10 fibrils for Hsp104Y507A, n = 16 fibrils for Hsp104Y257A in 2 observations for each condition, and mean ± S.D. values are shown. Statistical significance was analyzed by unpaired one-way ANOVA. **** P < 0.0001.
Extended Data Fig. 7 Sc37 amyloid is less efficiently fragmented by the three chaperones.
a, Representative AFM images of 0.5 μM Sc37 amyloid after incubation with 1 μM Hsp104, 2 μM Ssa1 and 2 μM Sis1 at 30 °C for 48 hours. b, Representative time-lapse TIRF images showing interactions between Sc37 amyloid-STELLA650, Hsp104-SNAP549 and Ssa1-Alexa488, after 1 μM Hsp104 including 30 nM Hsp104-SNAP549, 2 μM Ssa1-Alexa488 and 0.5 μM Sis1 were mixed and flowed. Images were acquired every 500 msec. Scale bar, 3 μm.
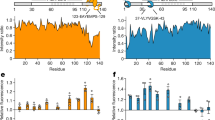
Extended Data Fig. 8 Fragmentation is frequently observed in Sc4 but not Sc37 amyloid.
a, Representative time-lapse TIRF images of Sc4 or Sc37 amyloid-STELLA650 and Hsp104-SNAP549, after 1 μM Hsp104 including 30 nM Hsp104-SNAP549, 2 μM Ssa1 and 2 μM Sis1 were mixed and flowed. Images were acquired every 500 msec. Scale bar, 5 μm. b, Representative intensity profiles of the spots in a 3×3 pixel area of Sc4 (top) or Sc37 (bottom) amyloid-STELLA650 (red) and Hsp104-SNAP549 (green) at the sites where Sc4/Sc37 amyloid was fragmented or non-fragmented. White arrows indicate the fragmentation of amyloid. 16 fibrils were analyzed from 2 observations for Sc4 amyloid and 8 fibrils were analyzed from 2 observations for Sc37 amyloid. c, Dwell time (T) of Hsp104 at the sites where Sc4 (left) or Sc37 (right) amyloid was fragmented or non-fragmented.
Extended Data Fig. 9 Hsp104trap is not continuously bound to Sc4 and Sc37 amyloid.
a, Representative time-lapse TIRF images of Sc4 or Sc37 amyloid-STELLA650 after 50 nM Hsp104trap-SNAP549, 2 μM Ssa1 and 2 μM Sis1 were mixed and flowed. Scale bar, 5 μm. b, Intensity profiles of Sc4 or Sc37 amyloid-STELLA650 and Hsp104trap-SNAP549 fluorescence in a 3×3 pixel area of Supplementary Fig. 3a from 0 to 600 sec. c, Dwell time of Hsp104trap-SNAP549 to Sc4 or Sc37 amyloid in Supplementary Fig. 3b. 12 fibrils were analyzed from 1 observation for Sc4 amyloid and 44 fibrils were analyzed from 4 observations for Sc37 amyloid. d, Number of Hsp104trap appearance on Sc4 and Sc37 amyloids was counted during 600 sec in a 3×3 pixel area. Mean ± S.D. values are shown in the dot plot. 12 fibrils were analyzed from 1 observation for Sc4 amyloid and 15 fibrils were analyzed from 1 observation for Sc37 amyloid. Statistical analysis was performed by the two-tailed Mann-Whitney U test.
Extended Data Fig. 10 Dissolution mode of Sc37 amyloid disaggregation is not altered by the low concentration of Ssa1.
a, Representative time-lapse TIRF images showing interactions between Sc37 amyloid-STELLA650, Hsp104-SNAP549 and Ssa1-Alexa488, after 1 μM Hsp104 including 30 nM Hsp104-SNAP549, 2 (top) or 0.5 (bottom) μM Ssa1-Alexa488 and 0.5 μM Sis1 were mixed and flowed. Images were acquired every 500 msec. Scale bar, 5 μm. b, Representative intensity profiles of the spots in a 3×3 pixel area of Sc37 amyloid-STELLA650 (red), Hsp104-SNAP549 (green) and Ssa1-Alexa488 (blue). c-d, Normalized cumulative fluorescence intensity of (c) Ssa1 and (d) Hsp104 over all 600 sec period in each Ssa1 concentration. Cumulative intensities of Hsp104 and Ssa1 were normalized by averaged intensities of Hsp104 and Ssa1 in the presence of 2 μM Ssa1, respectively. e, Analysis of fragmentation rate of Sc37 amyloid. Each data point shows the cumulative number of fragmentation events (per μm amyloid) at various time points. Data were averaged from 6 independent fibers (n = 5) in 1 observation. Error bars indicate S.D. f, Fluorescence intensity of Sc37 amyloid in a 3×3 pixel area was measured at 600 sec and normalized to that in the same area measured at 0 sec in the presence of 0.5 or 2 μM Ssa1 (n = 50 for 0.5 μM Ssa1, n = 42 for 2 μM Ssa1). g, Number of Hsp104 appearance on Sc37 amyloid during 600 sec in each Ssa1 concentration. In c-d and f-g, independent 3×3 pixel areas (n = 50 for 0.5 μM Ssa1, n = 42 for 2 μM Ssa1) were analyzed from 4 fibrils in 1 observation for each condition, and mean ± S.D. values are shown. Statistical significance was analyzed by the two-tailed Mann-Whitney U test. **** P < 0.0001. h, A model of two distinct, strain conformation-dependent modes of amyloid disaggregation. In Sc4 amyloid, Ssa1 and Sis1 bind to the amyloid, then recruit Hsp104. Following repeated binding of Hsp104 to the same site of amyloid results in ‘fragmentation’ of Sc4 amyloid. On the other hand, in Sc37 amyloid, enhanced accumulation of both Ssa1 and Sis1 recruits more Hsp104 proteins to the amyloid, resulting in less frequent repeated binding of Hsp104 and ‘dissolution’ of Sc37 amyloid.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3, video legends and Data 1–3.
Supplementary Video 1
Real-time TIRF imaging analysis of disaggregation of Sc4 amyloid by Hsp104, Ssa1 and Sis1.
Supplementary Video 2
Dual-color TIRF imaging analysis of Hsp104 binding to Sc4 amyloid.
Supplementary Video 3
Dual-color TIRF imaging analysis of Ssa1 binding to Sc4 amyloid.
Supplementary Video 4
Three-color TIRF imaging analysis of Ssa1 and Sis1 binding to Sc4 amyloid.
Supplementary Video 5
Three-color TIRF imaging analysis of Hsp104 and Ssa1 binding to Sc4 amyloid.
Supplementary Data 1
Disaggregation activity of fluorescently labeled chaperones.
Supplementary Data 2
Characterization of Hsp104trap mutant.
Supplementary Data 3
Morphological analyses of Sc4 and Sc37 amyloid fibrils.
Source data
Source Data Fig. 1
Numerical source data, unprocessed AFM images in Fig. 1c and unprocessed western blots in Fig. 1d.
Source Data Fig. 2
Numerical source data and TIRF images of Sc4 amyloid–STELLA650, Sc4 amyloid–STELLA650 + Hsp104, Sc4 amyloid–STELLA650 + Hsp104 + Ssa1, Sc4 amyloid–STELLA650 + Hsp104 + Ssa1 + Sis1 and Sc4 amyloid–STELLA650 + Hsp104KT + Ssa1 + Sis1 at 0, 50, 100, 150, 200, 250 and 300 s in Fig. 2a.
Source Data Fig. 3
Numerical source data, TIRF images of Sc4 amyloid–STELLA650, Ssa1–Alexa488 and Sis1–Cy3 at 0–800 s in Fig. 3a and TIRF images of Sc4 amyloid–STELLA650, Hsp104–SNAP549 and Ssa1–Alexa488 at 0–900 s in Fig. 3e.
Source Data Fig. 4
Numerical source data and TIRF images of Sc4 amyloid–STELLA650, Hsp104–SNAP549 and Ssa1–Alexa488 at 0–600 s in Fig. 4a.
Source Data Fig. 5
Numerical source data and TIRF images of Sc37 amyloid–STELLA650, Sc37 amyloid–STELLA650 + Hsp104, Sc37 amyloid–STELLA650 + Ssa1 + Sis1 and Sc37 amyloid–STELLA650 + Hsp104 + Ssa1 + Sis1 at 0, 100, 200, 400 and 900 s in Fig. 5b.
Source Data Fig. 6
Numerical source data, super-resolution microscopy images of Sc4 amyloid–STELLA650 + Ssa1–Alexa488 + Sis1 and Sc37 amyloid–STELLA650 + Ssa1–Alexa488 + Sis1 in Fig. 6g.
Source Data Extended Data Fig. 1
Numerical source data, unprocessed gel of Hsp104, Ssa1, Sis1 and Ydj1 in Extended Data Fig.1a, unprocessed AFM image of Sup35NM amyloid at 0, 15, 30, 45 min in Extended Data Fig.1c and unprocessed AFM image of Sup35NM amyloid of Sup35NM only, Sup35NM + Hsp104/Ssa1/Sis1 and Sup35NM + Hsp104KT/Ssa1/Sis1 in Extended Data Fig. 1d.
Source Data Extended Data Fig. 2
Numerical source data and unprocessed western blot in Extended Data Fig. 2d.
Source Data Extended Data Fig. 3
Numerical source data and TIRF images of Sc4 amyloid–STELLA650 + Hsp104–SNAP549, Sc4 amyloid–STTELA650 + Hsp104–SNAP549 + Ssa1, Sc4 amyloid–STELLA650 + Hsp104–SNAP549 + Sis1, Sc4 amyloid–STELLA650 + Hsp104–SNAP549 + Ssa1 + Sis1 and Sc4 amyloid–STELLA650 + Hsp104–SNAP549 + Ssa1 + Sis1 in ATPγS at 0, 100, 200, 300, 400, 500 and 600 s in Extended Data Fig. 3b.
Source Data Extended Data Fig. 4
Numerical source data, TIRF images of Sc4 amyloid–STELLA650 + Ssa1–Alexa488 in ATP, Sc4 amyloid–STELLA650 + Ssa1–Alexa488 + Sis1 in ATP, Sc4 amyloid–STELLA650 + Ssa1–Alexa488 + Sis1 in ATPγS, Sc4 amyloid–STELLA650 + Ssa1–Alexa488 in ADP and Sc4 amyloid–STELLA650 + Ssa1–Alexa488 + Sis1 in ADP at 0–1,200 s in Extended Data Fig.4a and TIRF images of Sc4 amyloid–STELLA650 + Ssa1 + Sis1–Cy3 in ATP, Sc4 amyloid–STELLA650 + Sis1–Cy3 in ATP and Sc4 amyloid–STELLA650 + Ssa1 + Sis1-Cy3 in ATPγS in Extended Data Fig. 4b.
Source Data Extended Data Fig. 5
Numerical source data.
Source Data Extended Data Fig. 6
Numerical source data, TIRF images of Sc4 amyloid–STELLA650 + Hsp104 WT/Ssa1/Sis1 at 0, 60, 120, 180, 240 and 300 s in Extended Data Fig. 6b, TIRF images of Sc4 amyloid–STELLA650 with Hsp104 Y507A/Ssa1/Sis1 or Hsp104 Y257A/Ssa1/Sis1 at 0, 200, 300, 400, 500 and 600 s in Extended Data Fig. 6b and TIRF images of Sc4 amyloid–STELLA650 with Hsp104 WT–SNAP549/Ssa1/Sis1, Hsp104 Y507A–SNAP549/Ssa1/Sis1 or Hsp104 Y257A–SNAP549/Ssa1/Sis1 at 0–150 s in Extended Data Fig. 6d.
Source Data Extended Data Fig. 7
Unprocessed AFM images of Sc37 amyloid in Extended Data Fig. 7a and TIRF images of Sc37 amyloid–STELLA650, Hsp104–SNAP549 and Ssa1–Alexa488 at 0–1,200 s in Extended Data Fig. 7b.
Source Data Extended Data Fig. 8
Numerical source data and TIRF images of Sc4 amyloid–STELLA650 + Hsp104–SNAP549 + Ssa1 + Sis1 and Sc37 amyloid–STELLA650 + Hsp104–SNAP549 + Ssa1 + Sis1 at 0, 100, 200, 300 and 400 s in Extended Data Fig. 8a.
Source Data Extended Data Fig. 9
Numerical source data and TIRF images of Sc4 amyloid–STELLA650 + Hsp104trap–SNAP549 + Ssa1 + Sis1 and Sc37 amyloid–STELLA650 + Hsp104trap–SNAP549 + Ssa1 + Sis1 at 0, 200, 400, 800 and 1,200 s in Extended Data Fig. 9a.
Source Data Extended Data Fig. 10
Numerical source data and TIRF image of Sc37 amyloid–STELLA650, Hsp104–SNAP549 and Ssa1–Alexa488 at 0, 200, 400, 600 and 900 s in Extended Data Fig. 10a.
Rights and permissions
About this article
Cite this article
Nakagawa, Y., Shen, H.CH., Komi, Y. et al. Amyloid conformation-dependent disaggregation in a reconstituted yeast prion system. Nat Chem Biol 18, 321–331 (2022). https://doi.org/10.1038/s41589-021-00951-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-021-00951-y
This article is cited by
-
MIL-CELL: a tool for multi-scale simulation of yeast replication and prion transmission
European Biophysics Journal (2023)
-
Picturing protein disaggregation
Nature Chemical Biology (2022)