Abstract
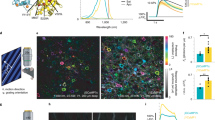
We describe single-component optogenetic probes whose activation dynamics depend on both light and temperature. We used the BcLOV4 photoreceptor to stimulate Ras and phosphatidyl inositol-3-kinase signaling in mammalian cells, allowing activation over a large dynamic range with low basal levels. Surprisingly, we found that BcLOV4 membrane translocation dynamics could be tuned by both light and temperature such that membrane localization spontaneously decayed at elevated temperatures despite constant illumination. Quantitative modeling predicted BcLOV4 activation dynamics across a range of light and temperature inputs and thus provides an experimental roadmap for BcLOV4-based probes. BcLOV4 drove strong and stable signal activation in both zebrafish and fly cells, and thermal inactivation provided a means to multiplex distinct blue-light sensitive tools in individual mammalian cells. BcLOV4 is thus a versatile photosensor with unique light and temperature sensitivity that enables straightforward generation of broadly applicable optogenetic tools.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All raw data used to generate the figures can be found at the following link: https://drive.google.com/drive/folders/1h0eDceDplxYUguSpUNyg5CHA4uudZO39?usp=sharing
Code availability
MATLAB code used to fit data and model BcLOV4 activation dynamics can be found at the following link: https://drive.google.com/drive/folders/1h0eDceDplxYUguSpUNyg5CHA4uudZO39?usp=sharing
References
Christie, J. M., Salomon, M., Nozue, K., Wada, M. & Briggs, W. R. LOV (light, oxygen, or voltage) domains of the blue-light photoreceptor phototropin (nph1): binding sites for the chromophore flavin mononucleotide. Proc. Natl Acad. Sci. USA 96, 8779–8783 (1999).
Schwerdtfeger, C. & Linden, H. VIVID is a flavoprotein and serves as a fungal blue light photoreceptor for photoadaptation. EMBO J. 22, 4846–4855 (2003).
Dietler, J. et al. A light-oxygen-voltage receptor integrates light and temperature. J. Mol. Biol. 433, 167107 (2021).
Hunt, S. M., Elvin, M., Crosthwaite, S. K. & Heintzen, C. The PAS/LOV protein VIVID controls temperature compensation of circadian clock phase and development in Neurospora crassa. Genes Dev 21, 1964–1974 (2007).
Gutiérrez-Medina, B. & Candia, C. N. H. Aggregation kinetics of the protein photoreceptor Vivid. Biochim. Biophys. Acta Proteins Proteom 1869, 140620 (2021).
Guntas, G. et al. Engineering an improved light-induced dimer (iLID) for controlling the localization and activity of signaling proteins. Proc. Natl Acad. Sci. USA 112, 112–117 (2015).
Kennedy, M. J. et al. Rapid blue-light-mediated induction of protein interactions in living cells. Nat. Methods 7, 973–975 (2010).
Shimizu-Sato, S., Huq, E., Tepperman, J. M. & Quail, P. H. A light-switchable gene promoter system. Nat. Biotechnol. 20, 1041–1044 (2002).
Strickland, D. et al. TULIPs: tunable, light-controlled interacting protein tags for cell biology. Nat. Methods 9, 379–384 (2012).
Kawano, F., Suzuki, H., Furuya, A. & Sato, M. Engineered pairs of distinct photoswitches for optogenetic control of cellular proteins. Nat. Commun. 6, 6256 (2015).
Zhou, X. X., Chung, H. K., Lam, A. J. & Lin, M. Z. Optical control of protein activity by fluorescent protein domains. Science 338, 810–814 (2012).
Wu, Y. I. et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature 461, 104–108 (2009).
Bugaj, L. J., Choksi, A. T., Mesuda, C. K., Kane, R. S. & Schaffer, D. V. Optogenetic protein clustering and signaling activation in mammalian cells. Nat. Methods 10, 249–252 (2013).
Boyden, E. S., Zhang, F., Bamberg, E., Nagel, G. & Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 8, 1263–1268 (2005).
Glantz, S. T. et al. Directly light-regulated binding of RGS-LOV photoreceptors to anionic membrane phospholipids. Proc. Natl Acad. Sci. USA 115, E7720–E7727 (2018).
He, L. et al. Optical control of membrane tethering and interorganellar communication at nanoscales. Chem. Sci. 8, 5275–5281 (2017).
McCubrey, J. A. et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim. Biophys. Acta 1773, 1263–1284 (2007).
Chang, F. et al. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia 17, 1263–1293 (2003).
Vanhaesebroeck, B., Stephens, L. & Hawkins, P. PI3K signalling: the path to discovery and understanding. Nat. Rev. Mol. Cell Biol. 13, 195–203 (2012).
Yang, J. et al. Targeting PI3K in cancer: mechanisms and advances in clinical trials. Mol. Cancer 18, 26 (2019).
Bugaj, L. J. et al. Cancer mutations and targeted drugs can disrupt dynamic signal encoding by the Ras-Erk pathway. Science 361, eaao3048 (2018).
Hino, N. et al. ERK-mediated mechanochemical waves direct collective cell polarization. Dev. Cell 53, 646–660.e8 (2020).
Johnson, H. E. et al. The spatiotemporal limits of developmental Erk signaling. Dev. Cell 40, 185–192 (2017).
Johnson, H. E. & Toettcher, J. E. Signaling dynamics control cell fate in the early Drosophila embryo. Dev. Cell 48, 361–370.e3 (2019).
Toettcher, J. E., Gong, D., Lim, W. A. & Weiner, O. D. Light-based feedback for controlling intracellular signaling dynamics. Nat. Methods 8, 837–839 (2011).
Toettcher, J. E., Weiner, O. D. & Lim, W. A. Using optogenetics to interrogate the dynamic control of signal transmission by the Ras/Erk module. Cell 155, 1422–1434 (2013).
Bugaj, L. J. & Lim, W. A. High-throughput multicolor optogenetics in microwell plates. Nat. Protoc. 14, 2205–2228 (2019).
Berlew, E. E., Kuznetsov, I. A., Yamada, K., Bugaj, L. J. & Chow, B. Y. Optogenetic Rac1 engineered from membrane lipid-binding RGS-LOV for inducible lamellipodia formation. Photochem. Photobiol. Sci. 19, 353–361 (2020).
Berlew, E. E. et al. Single‐component optogenetic tools for inducible RhoA GTPase signaling. Adv. Biol. (Weinh) 5, 2100810 (2021).
Hannanta-Anan, P., Glantz, S. T. & Chow, B. Y. Optically inducible membrane recruitment and signaling systems. Curr. Opin. Struct. Biol. 57, 84–92 (2019).
Suh, B.-C., Inoue, T., Meyer, T. & Hille, B. Rapid chemically induced changes of PtdIns(4,5)P2 gate KCNQ ion channels. Science 314, 1454–1457 (2006).
Regot, S., Hughey, J. J., Bajar, B. T., Carrasco, S. & Covert, M. W. High-sensitivity measurements of multiple kinase activities in live single cells. Cell 157, 1724–1734 (2014).
Mayr, V., Sturtzel, C., Stadler, M., Grissenberger, S. & Distel, M. Fast dynamic in vivo monitoring of Erk activity at single cell resolution in DREKA zebrafish. Front. Cell Dev. Biol. 6, 111 (2018).
Adrian, M., Nijenhuis, W., Hoogstraaten, R. I., Willems, J. & Kapitein, L. C. A phytochrome-derived photoswitch for intracellular transport. ACS Synth. Biol. 6, 1248–1256 (2017).
Golic, A. E. et al. BlsA is a low to moderate temperature blue light photoreceptor in the human pathogen Acinetobacter baumannii. Front. Microbiol. 10, 1925 (2019).
Nakasone, Y., Ono, T.-A., Ishii, A., Masuda, S. & Terazima, M. Temperature-sensitive reaction of a photosensor protein YcgF: possibility of a role of temperature sensor. Biochemistry 49, 2288–2296 (2010).
Nakasone, Y. et al. Stability of dimer and domain–domain interaction of Arabidopsis phototropin 1 LOV2. J. Mol. Biol. 383, 904–913 (2008).
Richter, F. et al. Engineering of temperature- and light-switchable Cas9 variants. Nucleic Acids Res. 44, 10003–10014 (2016).
Benedetti, L. et al. Optimized Vivid-derived magnets photodimerizers for subcellular optogenetics. eLife 9, e63230 (2020).
Hernández-Candia, C. N., Casas-Flores, S. & Gutiérrez-Medina, B. Light induces oxidative damage and protein stability in the fungal photoreceptor Vivid. PLoS ONE 13, e0201028 (2018).
Van Brederode, M. E., Hoff, W. D., Van Stokkum, I. H., Groot, M. L. & Hellingwerf, K. J. Protein folding thermodynamics applied to the photocycle of the photoactive yellow protein. Biophys. J. 71, 365–380 (1996).
Benzinger, D., Ovinnikov, S. & Khammash, M. Synthetic gene networks recapitulate dynamic signal decoding and differential gene expression. bioRxiv 2021.01.07.425755 (2021). https://doi.org/10.1101/2021.01.07.425755
Duan, L. et al. Understanding CRY2 interactions for optical control of intracellular signaling. Nat. Commun. 8, 547 (2017).
Fukaya, T., Lim, B. & Levine, M. Enhancer control of transcriptional bursting. Cell 166, 358–368 (2016).
Santos, M. G., Jorge, S. A. C., Brillet, K. & Pereira, C. A. Improving heterologous protein expression in transfected Drosophila S2 cells as assessed by EGFP expression. Cytotechnology 54, 15–24 (2007).
Villefranc, J. A., Amigo, J. & Lawson, N. D. Gateway compatible vectors for analysis of gene function in the zebrafish. Dev. Dyn. 236, 3077–3087 (2007).
Lamprecht, M. R., Sabatini, D. M. & Carpenter, A. E. CellProfilerTM: free, versatile software for automated biological image analysis. Biotechniques 42, 71–75 (2007).
Wickham, H., François, R., Henry, L. & Müller, K. dplyr: a grammar of data manipulation. R package v.0.8. 0.1 https://CRAN.R-project.org/package=dplyr (2019).
Wickham, H. ggplot2: ggplot2. Wiley Interdiscip. Rev. Comput. Stat. 3, 180–185 (2011).
Berg, S. et al. ilastik: interactive machine learning for (bio)image analysis. Nat. Methods 16, 1226–1232 (2019).
Acknowledgements
We thank C. Seiler and the CHOP Aquatic Zebrafish Core for assistance in injecting and mounting zebrafish embryos, D. Wu and D. Chenoweth for assistance with TIRF microscopy and H. Johnson and J. Toettcher (Princeton) for providing Drosophila S2 cells. This work was supported by the National Institutes of Health (R35GM138211, R21GM132831 for L.J.B., R35GM133425 for H.D. and B.L., R01NS101106 for E.E.B and B.Y.C. and R01HL152086 to A.F.S.) and the National Science Foundation (Graduate Research Fellowship Program to W.B., CAREER MCB1652003 for E.E.B. and B.Y.C.).
Author information
Authors and Affiliations
Contributions
W.B., B.Y.C. and L.J.B. conceived the study. W.B., E.E.B., H.D., I.A.K., A.F.S., C.P. and L.J.B. performed experiments and analyzed data. A.F.S., B.L., B.Y.C. and L.J.B. supervised the work. W.B. and L.J.B. wrote the manuscript and made figures, with editing from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Chemical Biology thanks Matias Zurbriggen, Maxwell Wilson and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figures 1–16 and movie captions.
Supplementary Movie 1
Temperature-dependent decay of BcLOV-mCh membrane localization. HEK 293 T cells expressing BcLOV-mCh were stimulated with 488 nm light and imaged over 40 min. Membrane localization was sustained at lower temperatures (left), but decayed at higher temperatures (right). Time is mm:ss.
Supplementary Movie 2
BcLOV-mCh translocation in zebrafish embryos. 24 hpf zebrafish embryos expressing BcLOV-mCh were stimulated with 488 nm light and imaged with confocal microscopy. Time is mm:ss.
Rights and permissions
About this article
Cite this article
Benman, W., Berlew, E.E., Deng, H. et al. Temperature-responsive optogenetic probes of cell signaling. Nat Chem Biol 18, 152–160 (2022). https://doi.org/10.1038/s41589-021-00917-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-021-00917-0
This article is cited by
-
Optogenetics in the hot seat
Nature Chemical Biology (2022)
-
Spatiotemporal control of necroptotic cell death and plasma membrane recruitment using engineered MLKL domains
Cell Death Discovery (2022)