Abstract
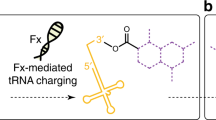
When the primitive translation system first emerged in the hypothetical RNA world, ribozymes could have been responsible for aminoacylation. Given that naturally occurring T-box riboswitches selectively sense the aminoacylation status of cognate tRNAs, we introduced a domain of random sequence into a T-box–tRNA conjugate and isolated ribozymes that were self-aminoacylating on the 3′-terminal hydroxyl group. One of them, named Tx2.1, recognizes the anticodon and D-loop of tRNA via interaction with its stem I domain, similarly to the parental T-box, and selectively charges N-biotinyl-l-phenylalanine (Bio-lPhe) onto the 3′ end of the cognate tRNA in trans. We also demonstrated the ribosomal synthesis of a Bio-lPhe-initiated peptide in a Tx2.1-coupled in vitro translation system, in which Tx2.1 catalyzed specific tRNA aminoacylation in situ. This suggests that such ribozymes could have coevolved with a primitive translation system in the RNA world.

This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All raw data are available from the corresponding author upon reasonable request.
References
Walter, G. Origin of life: the RNA world. Nature 319, 618 (1986).
Yarus, M. A specific amino acid binding site composed of RNA. Science 240, 1751–1758 (1988).
Schimmel, P. The RNP bridge between two worlds. Nat. Rev. Mol. Cell Biol. 12, 135 (2011).
Kruger, K. et al. Self-splicing RNA: autoexcision and autocyclization of the ribosomal RNA intervening sequence of Tetrahymena. Cell 31, 147–157 (1982).
Guerrier-Takada, C., Gardiner, K., Marsh, T., Pace, N. & Altman, S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell 35, 849–857 (1983).
Robertson, M. P. & Joyce, G. F. Highly efficient self-replicating RNA enzymes. Chem. Biol. 21, 238–245 (2014).
Nissen, P., Hansen, J., Ban, N., Moore, P. B. & Steitz, T. A. The structural basis of ribosome activity in peptide bond synthesis. Science 289, 920–930 (2000).
Saito, H., Kourouklis, D. & Suga, H. An in vitro evolved precursor tRNA with aminoacylation activity. EMBO J. 20, 1797–1806 (2001).
Murakami, H., Saito, H. & Suga, H. A versatile tRNA aminoacylation catalyst based on RNA. Chem. Biol. 10, 655–662 (2003).
Murakami, H., Ohta, A., Ashigai, H. & Suga, H. A highly flexible tRNA acylation method for non-natural polypeptide synthesis. Nat. Methods 3, 357–359 (2006).
Xiao, H., Murakami, H., Suga, H. & Ferré-D’Amaré, A. R. Structural basis of specific tRNA aminoacylation by a small in vitro selected ribozyme. Nature 454, 358–361 (2008).
Niwa, N., Yamagishi, Y., Murakami, H. & Suga, H. A flexizyme that selectively charges amino acids activated by a water-friendly leaving group. Bioorg. Med. Chem. Lett. 19, 3892–3894 (2009).
Futai, K., Terasaka, N., Katoh, T. & Suga, H. tRid, an enabling method to isolate previously inaccessible small RNA fractions. Methods 106, 105–111 (2016).
Ohta, A., Murakami, H., Higashimura, E. & Suga, H. Synthesis of polyester by means of genetic code reprogramming. Chem. Biol. 14, 1315–1322 (2007).
Goto, Y. et al. Reprogramming the translation initiation for the synthesis of physiologically stable cyclic peptides. ACS Chem. Biol. 3, 120–129 (2008).
Kawakami, T. et al. Diverse backbone-cyclized peptides via codon reprogramming. Nat. Chem. Biol. 5, 888–890 (2009).
Kawakami, T., Murakami, H. & Suga, H. Messenger RNA-programmed incorporation of multiple N-methyl-amino acids into linear and cyclic peptides. Chem. Biol. 15, 32–42 (2008).
Grundy, F. J., Rollins, S. M. & Henkin, T. M. Interaction between the acceptor end of tRNA and the T box stimulates antitermination in the Bacillus subtilis tyrS gene: a new role for the discriminator base. J. Bacteriol. 176, 4518–4526 (1994).
Grundy, F. J. & Henkin, T. M. tRNA as a positive regulator of transcription antitermination in B. subtilis. Cell 74, 475–482 (1993).
Grundy, F. J., Winkler, W. C. & Henkin, T. M. tRNA-mediated transcription antitermination in vitro: codon–anticodon pairing independent of the ribosome. Proc. Natl Acad. Sci. USA 99, 11121–11126 (2002).
Yousef, M. R., Grundy, F. J. & Henkin, T. M. tRNA requirements for glyQS antitermination: a new twist on tRNA. RNA 9, 1148–1156 (2003).
Grigg, J. C. et al. T box RNA decodes both the information content and geometry of tRNA to affect gene expression. Proc. Natl Acad. Sci. USA 110, 7240–7245 (2013).
Zhang, J. & Ferré-D’Amaré, A. R. Co-crystal structure of a T-box riboswitch stem I domain in complex with its cognate tRNA. Nature 500, 363–366 (2013).
Grigg, J. C. & Ke, A. Structural determinants for geometry and information decoding of tRNA by T box leader RNA. Structure 21, 2025–2032 (2013).
Li, S. et al. Structural basis of amino acid surveillance by higher-order tRNA–mRNA interactions. Nat. Struct. Mol. Biol. 26, 1094–1105 (2019).
Zhang, J. & Ferré-D’Amaré, A. R. Direct evaluation of tRNA aminoacylation status by the T-box riboswitch using tRNA–mRNA stacking and steric readout. Mol. Cell 55, 148–155 (2014).
Suga, H., Lohse, P. A. & Szostak, J. W. Structural and kinetic characterization of an acyl transferase ribozyme. J. Am. Chem. Soc. 120, 1151–1156 (1998).
Lee, N., Bessho, Y., Wei, K., Szostak, J. W. & Suga, H. Ribozyme-catalyzed tRNA aminoacylation. Nat. Struct. Mol. Biol. 7, 28–33 (2000).
Tuerk, C. & Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 249, 505–510 (1990).
Ellington, A. & Szostak, J. In vitro selection of RNA molecules that bind specific ligands. Nature 346, 818–822 (1990).
Robertson, D. & Joyce, G. Selection in vitro of an RNA enzyme that specifically cleaves single-stranded DNA. Nature 344, 467–468 (1990).
Lohse, P. A. & Szostak, J. W. Ribozyme-catalysed amino-acid transfer reactions. Nature 381, 442–444 (1996).
Köhrer, C. & Rajbhandary, U. The many applications of acid urea polyacrylamide gel electrophoresis to studies of tRNAs and aminoacyl-tRNA synthetases. Methods 44, 129–138 (2008).
Caserta, E., Liu, L. C., Grundy, F. J. & Henkin, T. M. Codon–anticodon recognition in the Bacillus subtilis glyQS T box riboswitch: RNA-dependent codon selection outside the ribosome. J. Biol. Chem. 290, 23336–23347 (2015).
Chan, P. P. & Lowe, T. M. GtRNAdb 2.0: an expanded database of transfer RNA genes identified in complete and draft genomes. Nucleic Acids Res. 44, D184–D189 (2016).
Kobori, S. & Yokobayashi, Y. High-throughput mutational analysis of a twister ribozyme. Angew. Chem. Int. Ed. Engl. 55, 10354–10357 (2016).
Dhamodharan, V., Kobori, S. & Yokobayashi, Y. Large scale mutational and kinetic analysis of a self-hydrolyzing deoxyribozyme. ACS Chem. Biol. 12, 2940–2945 (2017).
Rogers, J. M., Passioura, T. & Suga, H. Nonproteinogenic deep mutational scanning of linear and cyclic peptides. Proc. Natl Acad. Sci. USA 115, 10959–10964 (2018).
Sato, K., Hamada, M., Asai, K. & Mituyama, T. CENTROIDFOLD: a web server for RNA secondary structure prediction. Nucleic Acids Res. 37, W277–W280 (2009).
Goto, Y., Katoh, T. & Suga, H. Flexizymes for genetic code reprogramming. Nat. Protoc. 6, 779–790 (2011).
Goto, Y., Iseki, M., Hitomi, A., Murakami, H. & Suga, H. Nonstandard peptide expression under the genetic code consisting of reprogrammed dual sense codons. ACS Chem. Biol. 8, 2630–2634 (2013).
Saito, H., Watanabe, K. & Suga, H. Concurrent molecular recognition of the amino acid and tRNA by a ribozyme. RNA 7, 1867–1878 (2001).
Saad, N. Y. et al. Two-codon T-box riboswitch binding two tRNAs. Proc. Natl Acad. Sci. USA 110, 12756–12761 (2013).
Vitreschak, A. G., Mironov, A. A., Lyubetsky, V. A. & Gelfand, M. S. Comparative genomic analysis of T-box regulatory systems in bacteria. RNA 14, 717–735 (2008).
Gutiérrez-Preciado, A., Henkin, T. M., Grundy, F. J., Yanofsky, C. & Merino, E. Biochemical features and functional implications of the RNA-based T-box regulatory mechanism. Microbiol. Mol. Biol. Rev. 73, 36–61 (2009).
Saito, H. & Suga, H. A ribozyme exclusively aminoacylates the 3′-hydroxyl group of the tRNA terminal adenosine. J. Am. Chem. Soc. 123, 7178–7179 (2001).
Saito, H. & Suga, H. Outersphere and innersphere coordinated metal ions in an aminoacyl-tRNA synthetase ribozyme. Nucleic Acids Res. 30, 5151–5159 (2002).
Suddala, K. C. & Zhang, J. An evolving tale of two interacting RNAs—themes and variations of the T-box riboswitch mechanism. IUBMB Life 71, 1167–1180 (2019).
Saad, N. Y. A ribonucleopeptide world at the origin of life. J. Syst. Evol. 56, 1–13 (2018).
Kao, C., Zheng, M. & Rüdisser, S. A simple and efficient method to reduce nontemplated nucleotide addition at the 3′ terminus of RNAs transcribed by T7 RNA polymerase. RNA 5, 1268–1272 (1999).
Acknowledgements
This work was supported by Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Research S (26220204), the Human Frontier Science Program (RGP0015/2017), the Japan Science and Technology (JST) agency and CREST-Molecular Technology (JPMJCR12L2) to H.S. N.T. is supported by Grants-in-Aid for JSPS Fellows (12J08188) and Grants-in-Aid for Early-Career Scientists (19K16200). S.I. was supported by Grants-in-Aid for JSPS Fellows (JP16J04031). T.K. is supported by a JSPS Grant-in-Aid for Challenging Exploratory Research (26560429).
Author information
Authors and Affiliations
Contributions
S.I. and N.T. conducted biochemical and chemical studies, and T.K. and H.S. supervised the research. All authors contributed to writing the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2 and Supplementary Figs. 1–12
Rights and permissions
About this article
Cite this article
Ishida, S., Terasaka, N., Katoh, T. et al. An aminoacylation ribozyme evolved from a natural tRNA-sensing T-box riboswitch. Nat Chem Biol 16, 702–709 (2020). https://doi.org/10.1038/s41589-020-0500-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-020-0500-6
This article is cited by
-
Development and characterization of a glycine biosensor system for fine-tuned metabolic regulation in Escherichia coli
Microbial Cell Factories (2022)
-
Structure and mechanism of the methyltransferase ribozyme MTR1
Nature Chemical Biology (2022)
-
A natural riboswitch scaffold with self-methylation activity
Nature Communications (2021)