Abstract
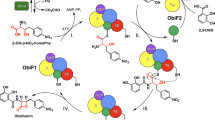
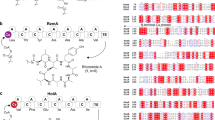
Nonribosomal depsipeptides are natural products composed of amino and hydroxy acid residues. The hydroxy acid residues often derive from α-keto acids, reduced by ketoreductase domains in the depsipeptide synthetases. Biochemistry and structures reveal the mechanism of discrimination for α-keto acids and a remarkable architecture: flanking intact adenylation and ketoreductase domains are sequences separated by >1,100 residues that form a split ‘pseudoAsub’ domain, structurally important for the depsipeptide module’s synthetic cycle.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Felnagle, E. A. et al. Nonribosomal peptide synthetases involved in the production of medically relevant natural products. Mol. Pharm. 5, 191–211 (2008).
Ekman, J. V. et al. Cereulide produced by Bacillus cereus increases the fitness of the producer organism in low-potassium environments. Microbiology 158, 1106–1116 (2012).
Alonzo, D. A., Magarvey, N. A. & Schmeing, T. M. Characterization of cereulide synthetase, a toxin-producing macromolecular machine. PLoS ONE 10, e0128569 (2015).
Jaitzig, J., Li, J., Sussmuth, R. D. & Neubauer, P. Reconstituted biosynthesis of the nonribosomal macrolactone antibiotic valinomycin in Escherichia coli. ACS Synth. Biol. 3, 432–438 (2014).
Huguenin-Dezot, N. et al. Trapping biosynthetic acyl-enzyme intermediates with encoded 2,3-diaminopropionic acid. Nature 565, 112–117 (2019).
Ding, Y., Rath, C. M., Bolduc, K. L., Hakansson, K. & Sherman, D. H. Chemoenzymatic synthesis of cryptophycin anticancer agents by an ester bond-forming non-ribosomal peptide synthetase module. J. Am. Chem. Soc. 133, 14492–14495 (2011).
Fujimori, D. G. et al. Cloning and characterization of the biosynthetic gene cluster for kutznerides. Proc. Natl Acad. Sci. USA 104, 16498–16503 (2007).
Ramaswamy, A. V., Sorrels, C. M. & Gerwick, W. H. Cloning and biochemical characterization of the hectochlorin biosynthetic gene cluster from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 70, 1977–1986 (2007).
Xu, Y. et al. Biosynthesis of the cyclooligomer depsipeptide bassianolide, an insecticidal virulence factor of Beauveria bassiana. Fungal Genet. Biol. 46, 353–364 (2009).
Magarvey, N. A., Ehling-Schulz, M. & Walsh, C. T. Characterization of the cereulide NRPS alpha-hydroxy acid specifying modules: activation of ɑ-keto acids and chiral reduction on the assembly line. J. Am. Chem. Soc. 128, 10698–10699 (2006).
Magarvey, N. A. et al. Biosynthetic characterization and chemoenzymatic assembly of the cryptophycins. Potent anticancer agents from cyanobionts. ACS Chem. Biol. 1, 766–779 (2006).
Cheng, Y. Q. Deciphering the biosynthetic codes for the potent anti-SARS-CoV cyclodepsipeptide valinomycin in Streptomyces tsusimaensis ATCC 15141. ChemBioChem 7, 471–477 (2006).
Ehling-Schulz, M. et al. Identification and partial characterization of the nonribosomal peptide synthetase gene responsible for cereulide production in emetic Bacillus cereus. Appl. Environ. Microbiol. 71, 105–113 (2005).
Ehling-Schulz, M. et al. Cereulide synthetase gene cluster from emetic Bacillus cereus: structure and location on a mega virulence plasmid related to Bacillus anthracis toxin plasmid pXO1. BMC Microbiol. 6, 20 (2006).
Geer, L. Y., Domrachev, M., Lipman, D. J. & Bryant, S. H. CDART: protein homology by domain architecture. Genome Res. 12, 1619–1623 (2002).
Mori, S. et al. Structural basis for backbone N-methylation by an interrupted adenylation domain. Nat. Chem. Biol. 14, 428–430 (2018).
Zheng, J., Piasecki, S. K. & Keatinge-Clay, A. T. Structural studies of an A2-type modular polyketide synthase ketoreductase reveal features controlling ɑ-substituent stereochemistry. ACS Chem. Biol. 8, 1964–1971 (2013).
Miller, B. R., Sundlov, J. A., Drake, E. J., Makin, T. A. & Gulick, A. M. Analysis of the linker region joining the adenylation and carrier protein domains of the modular nonribosomal peptide synthetases. Proteins 82, 2691–2702 (2014).
Conti, E., Stachelhaus, T., Marahiel, M. A. & Brick, P. Structural basis for the activation of phenylalanine in the non-ribosomal biosynthesis of gramicidin S. EMBO J. 16, 4174–4183 (1997).
Stachelhaus, T., Mootz, H. D. & Marahiel, M. A. The specificity-conferring code of adenylation domains in nonribosomal peptide synthetases. Chem. Biol. 6, 493–505 (1999).
Challis, G. L., Ravel, J. & Townsend, C. A. Predictive, structure-based model of amino acid recognition by nonribosomal peptide synthetase adenylation domains. Chem. Biol. 7, 211–224 (2000).
Allen, F. H., Baalham, C. A., Lommerse, J. P. M. & Raithby, P. R. Carbonyl–carbonyl interactions can be competitive with hydrogen bonds. Acta Crystallogr. Sect. B Struct. Sci. 54, 320–329 (1998).
Deane, C. M., Allen, F. H., Taylor, R. & Blundell, T. L. Carbonyl–carbonyl interactions stabilize the partially allowed Ramachandran conformations of asparagine and aspartic acid. Protein Eng. 12, 1025–1028 (1999).
Tanovic, A., Samel, S. A., Essen, L. O. & Marahiel, M. A. Crystal structure of the termination module of a nonribosomal peptide synthetase. Science 321, 659–663 (2008).
Reimer, J. M. et al. Structures of a dimodular nonribosomal peptide synthetase reveal conformational flexibility. Science 366, eaaw4388 (2019).
Chalut, C., Botella, L., de Sousa-D’Auria, C., Houssin, C. & Guilhot, C. The nonredundant roles of two 4’-phosphopantetheinyl transferases in vital processes of Mycobacteria. Proc. Natl Acad. Sci. USA 103, 8511–8516 (2006).
Reimer, J. M., Aloise, M. N., Harrison, P. M. & Schmeing, T. M. Synthetic cycle of the initiation module of a formylating nonribosomal peptide synthetase. Nature 529, 239–242 (2016).
Liu, Y. & Bruner, S. D. Rational manipulation of carrier-domain geometry in nonribosomal peptide synthetases. ChemBioChem 8, 617–621 (2007).
Nazi, I., Koteva, K. P. & Wright, G. D. One-pot chemoenzymatic preparation of coenzyme A analogues. Anal. Biochem. 324, 100–105 (2004).
D’Arcy, A., Bergfors, T., Cowan-Jacob, S. W. & Marsh, M. Microseed matrix screening for optimization in protein crystallization: what have we learned? Acta Crystallogr. F Struct. Biol. Commun. 70, 1117–1126 (2014).
Kabsch, W. XDS. Acta Crystallogr. D Biol. Crystallogr. 66, 125–132 (2010).
Biasini, M. et al. SWISS-MODEL: modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 42, W252–W258 (2014).
McCoy, A. J. et al. Phaser crystallographic software. J. Appl. Crystallogr. 40, 658–674 (2007).
Emsley, P., Lohkamp, B., Scott, W. G. & Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 66, 486–501 (2010).
Kleywegt, G. J. & Jones, T. A. in From First Map to Final Model (eds Bailey, S. et al.) 59–66 (SERC Daresbury Laboratory, 1994).
Murshudov, G. N. et al. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr. D Biol. Crystallogr. 67, 355–367 (2011).
Winter, G. et al. DIALS: implementation and evaluation of a new integration package. Acta Crystallogr. D Struct. Biol. 74, 85–97 (2018).
Battye, T. G. G., Kontogiannis, L., Johnson, O., Powell, H. R. & Leslie, A. G. W. iMOSFLM: a new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr. D Biol. Crystallogr. 67, 271–281 (2011).
Evans, P. R. & Murshudov, G. N. How good are my data and what is the resolution? Acta Crystallogr. D Biol. Crystallogr. 69, 1204–1214 (2013).
Adams, P. D. et al. Advances, interactions, and future developments in the CNS, Phenix, and Rosetta structural biology software systems. Annu. Rev. Biophys. 42, 265–287 (2013).
Wilson, D. J. & Aldrich, C. C. A continuous kinetic assay for adenylation enzyme activity and inhibition. Anal. Biochem. 404, 56–63 (2010).
Johnson, M. et al. NCBI BLAST: a better web interface. Nucleic Acids Res. 36, W5–W9 (2008).
Sievers, F. et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 7, 539 (2011).
Drozdetskiy, A., Cole, C., Procter, J. & Barton, G. J. JPred4: a protein secondary structure prediction server. Nucleic Acids Res. 43, W389–W394 (2015).
Acknowledgements
We thank C. Alonso for TEV protease purification and other laboratory assistance, J. Reimer for preparing amino coenzyme A, members of the Schmeing laboratory for helpful advice and discussion, N. Rogerson for proofreading, staff at APS (F. Murphy and S. Banarjee) and CLS for support during X-ray data collection and N. Magarvey for discussions and suggesting structural work on depsipeptide synthetases. This work was supported by a Canada Research Chair and NSERC Discovery Grant no. 418420 to T.M.S.
Author information
Authors and Affiliations
Contributions
T.M.S., D.A.A. and C.C.-L. designed the study and wrote the manuscript. D.A.A., C.C.-L. and M.J.T. performed biochemical experiments. J.W. performed structure determination and refinement of the A–KR structure using NCS averaging and map sharpening. D.A.A. and C.C.-L. performed crystallization, structure determination and refinement.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Tables 1 and 2 and Figs. 1–8
Rights and permissions
About this article
Cite this article
Alonzo, D.A., Chiche-Lapierre, C., Tarry, M.J. et al. Structural basis of keto acid utilization in nonribosomal depsipeptide synthesis. Nat Chem Biol 16, 493–496 (2020). https://doi.org/10.1038/s41589-020-0481-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41589-020-0481-5
This article is cited by
-
Catalytic trajectory of a dimeric nonribosomal peptide synthetase subunit with an inserted epimerase domain
Nature Communications (2022)
-
Structures and function of a tailoring oxidase in complex with a nonribosomal peptide synthetase module
Nature Communications (2022)