Abstract
Gray leaf spot (GLS), caused by the fungal pathogens Cercospora zeae-maydis and Cercospora zeina, is a major foliar disease of maize worldwide (Zea mays L.). Here we demonstrate that ZmWAKL encoding cell-wall-associated receptor kinase-like protein is the causative gene at the major quantitative disease resistance locus against GLS. The ZmWAKLY protein, encoded by the resistance allele, can self-associate and interact with a leucine-rich repeat immune-related kinase ZmWIK on the plasma membrane. The ZmWAKLY/ZmWIK receptor complex interacts with and phosphorylates the receptor-like cytoplasmic kinase (RLCK) ZmBLK1, which in turn phosphorylates its downstream NADPH oxidase ZmRBOH4. Upon pathogen infection, ZmWAKLY phosphorylation activity is transiently increased, initiating immune signaling from ZmWAKLY, ZmWIK, ZmBLK1 to ZmRBOH4, ultimately triggering a reactive oxygen species burst. Our study thus uncovers the role of the maize ZmWAKL–ZmWIK–ZmBLK1–ZmRBOH4 receptor/signaling/executor module in perceiving the pathogen invasion, transducing immune signals, activating defense responses and conferring increased resistance to GLS.
Similar content being viewed by others
Main
Plants have evolved multiple, varied signal reception/transduction mechanisms to control cellular functions and coordinate defense responses at the cellular, tissue and organismal levels. The initial perception of pathogen infection is mediated by pattern-recognition receptors (PRRs) at the plasma membrane, which include receptor-like kinases (RLKs) and receptor-like proteins1,2. The detection of pathogen-associated molecular patterns and damage-associated molecular patterns by cognate PRRs leads to pattern-triggered immunity (PTI), which includes rapid reactive oxygen species (ROS) production, calcium (Ca2+) influx, activation of calcium-dependent and mitogen-activated kinases, changes of immune-related gene expression and, in some cases, localized cell death3,4,5. PTI is vital for preventing infection of most nonadapted microbes and restricting the growth of adapted microbes, termed basal resistance6.
Cell-wall-associated kinases (WAKs) and WAK-like kinases (WAKLs) represent a unique class of RLKs that are major regulators of fungal disease resistance in plant species. In Arabidopsis, WAKL22/RFO1 confers resistance to a broad spectrum of Fusarium races7. The maize gene ZmWAK is induced specifically upon Sporisorium reilianum infection in the mesocotyl to inhibit hyphal growth8. ZmWAK-RLK1 confers resistance to northern corn leaf blight caused by Exserohilum turcicum9. Other WAK genes, such as Xa4 in rice, TaWAKL4 and Snn1 in wheat, also confer resistance to fungal diseases10,11,12.
Gray leaf spot (GLS) is a major foliar disease of maize caused by the fungal pathogens Cercospora zeae-maydis and Cercospora zeina13. Since its discovery in the United States in the 1920s, GLS has become a severe global maize disease. GLS was reported to cause substantial yield loss in susceptible cultivars14. In the northern United States and Ontario from 2016 to 2019, GLS caused the greatest estimated yield losses of any maize disease15. GLS resistance is inherited as a typical quantitative trait, and more than 100 quantitative trait loci (QTLs) have been identified across a range of studies14,16,17,18,19. So far, ZmCCoAOMT2 (caffeoyl-CoA O-methyltransferase 2) and ZmMM1 (Mexicana lesion mimic 1) are the only two genes identified to be effective against GLS20,21.
Here we report the map-based cloning of the GLS quantitative disease resistance (QDR) gene ZmWAKL and the elucidation of the signal transduction pathway, which activates defense responses against GLS in maize.
Fine-mapping of qRgls1 against GLS
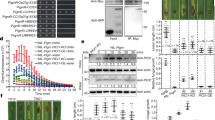
We previously mapped the major QDR locus qRgls1 to the short arm of chromosome 8 in a segregating population derived from a cross between the highly GLS-resistant inbred line Y32 and GLS-susceptible inbred line Q11. qRgls1 was fine-mapped to a 1.4-Mb interval. The Y32 allele at qRgls1 enhanced resistance by 19.7–61.3% compared to the Q11 allele22. Here we identified further recombinants in the qRgls1 region with which we fine-mapped qRgls1 to a 122-kb interval between markers IDP2 and M2 (B73_RefGen_v4; Extended Data Fig. 1). We allowed five key BC8F7 recombinants to self-pollinate to develop homozygous lines with or without Y32 segments. The Y32 donor segments resulted in significantly reduced levels of GLS in recombinants II, III and IV, but not in recombinants I and V, further confirming the location of qRgls1 (Fig. 1a). The Y32 allele at qRgls1 reduced the GLS disease severity index (DSI) significantly by 14.7–21.6% (Extended Data Fig. 2a).
a, Recombinant-derived homozygous lines with/without Y32 segments and their GLS-resistance performance (n = 1151). The symbols ‘+’/‘−’ indicate the presence/absence of the resistance allele at qRgls1, respectively. b, Schematic diagram of the complementary pCAMBIA3301-ZmWAKLY construct. c–h, Resistance performance of the complementation transgenic lines in self-pollinated (c–e) and T1BC1F1 and T1BC3F1 backcrossed populations (f–h). c,f, Relative expression of ZmWAKL was determined by RT–qPCR (n = 3), and the transgene was confirmed by PCR. d,e, GLS symptoms (d) and scales (e) of B73 and homozygous transgenic lines (n = 297). g,h, DSI values of transgenic and nontransgenic plants in T1BC1F1 (n = 189) and T1BC3F1 (n = 304) segregating populations. i, Schematic diagram of the overexpressed Ubipro:ZmWAKLY-GFP construct. j–o, GLS resistance of the overexpression transgenic plants in self-pollinated (j–l) and backcrossed populations (m–o). j,m, Relative expression of ZmWAKL was determined by RT–qPCR (n = 3), and the transgene was confirmed by immunoblotting with α-GFP antibody. k,l, GLS symptoms (k) and scales (l) of B73 and homozygous transgenic lines (n = 491). n,o, DSI values of transgenic and nontransgenic plants in T1BC1F1 (n = 469) and T1BC3F1 (n = 805) segregating populations. Data are presented as mean values in g and n; means ± s.e. in h and o; means ± s.d. in c, f, j and m. Data in a, e and l are displayed as box and whisker plots with individual data points, the asterisk denotes the mean and the box limits indicate the interquartile range. In c, e, j and l, different lowercase letters indicate a significant difference (P < 0.05) based on one-way ANOVA with Tukey’s test (c,j) or Fisher’s LSD test (e,l). In a, f, g, m and n, statistical significance was determined by a two-sided Student’s t test. Statistical significance was determined by a paired t test in h and o. Scale bars in a, d and k, 15 cm. LB and RB, the left and right T-DNA borders, respectively; NOS, nopaline synthase.
We selected two homozygous lines derived from recombinant IV, which shared a similar genetic background, but differed at qRgls1, named these near-isogenic lines NIL-Y32 (with the Y32 allele) and NIL-Q11 (with the Q11 allele; Fig. 1a). NIL-Y32 was more resistant to C. zeina than NIL-Q11 at 44 d postinoculation (dpi; Extended Data Fig. 2b,c). C. zeae-maydis and C. zeina conidiophores erupt through the stomata during infection13,23. Here scanning electron microscopy showed that there was much more profuse conidiation on NIL-Q11 compared to NIL-Y32 (Extended Data Fig. 2d). In several previous studies, GLS resistance has been associated with increased time to flowering14,24. These two NILs showed no significant differences in three flowering-related traits when grown under long-day or short-day conditions (Extended Data Fig. 2e,f).
We identified, sequenced and annotated two overlapping bacterial artificial chromosome (BAC) clones from the resistant parental line Y32 encompassing the qRgls1 region (Supplementary Fig. 1a,b). The qRgls1 interval was ~122 kb in B73 (B73_RefGen_v4) but only ~60 kb in Y32. The three predicted genes within the Y32 qRgls1 region were all shared with B73, encoded two cell-wall-associated receptor kinase-like proteins (Zm00001d008457, hereinafter referred to as ZmPR5L; Zm00001d008458, hereinafter referred to as ZmWAKL) and a hypothetical protein (Zm00001d008459). The B73 qRgls1 region additionally harbored a gene encoding a leucine-rich repeat RLK (Zm00001d008460; Fig. 1a and Supplementary Fig. 1b). We can’t detect expressions of either Zm00001d008459 or Zm00001d008460 by RT–qPCR; however, expressions of both ZmPR5L and ZmWAKL were detected, and these genes were considered candidates for qRgls1 (Supplementary Fig. 1c).
We determined the full-size complementary DNA (cDNA) sequence of ZmWAKL by rapid amplification of cDNA ends and amplified the full-size cDNA of ZmPR5L by RT–PCR (Supplementary Fig. 1d,e). We designated the Y32 alleles ZmWAKLY and ZmPR5LY and the Q11 alleles ZmWAKLQ and ZmPR5LQ. ZmWAKLY differs from ZmWAKLQ predominantly in the extracellular domain (ECD) with only 62.8% sequence identity. The intracellular domain (ICD) is more conserved with 92.9% sequence identity. Both ZmWAKL isoforms are non-arginine-aspartate (non-RD) kinases comprising the extracellular galacturonan-binding domain (GUB), transmembrane (TM) and cytoplasmic serine/threonine kinase (STK) domains (Extended Data Fig. 3a and Supplementary Fig. 2a). By contrast, ZmPR5LY and ZmPR5LQ shared 98.4% sequence identity (Extended Data Fig. 3b and Supplementary Fig. 2b).
ZmWAKL is the causal gene underlying qRgls1
We isolated a 9.2-kb Y32 genomic fragment comprising the native ZmWAKLY allele for the complementation test (Fig. 1b). We independently obtained five transgenic events in the background of the GLS-susceptible line B73. We self-pollinated three of them to develop homozygous transgenic lines expressing ZmWAKLY that had significantly higher GLS resistance than B73 (Fig. 1c–e). Notably, the enhanced GLS resistance did not cause substantial changes in plant architecture or flowering time (Supplementary Fig. 3a,b). We backcrossed another two transgenic events to Q11 to produce T1BC1F1 and T1BC3F1 populations. Transgenic plants consistently exhibited significantly lower DSI than their nontransgenic siblings (Fig. 1f–h). These results indicate that the ZmWAKLY allele confers resistance to GLS.
We also generated ten and three independent transgenic events overexpressing ZmWAKLY-GFP (GFP, green fluorescent protein) and ZmWAKLQ-GFP in the B73 background, respectively (Fig. 1i and Extended Data Fig. 4a). ZmWAKL-GFP was detectable by immunoblotting with α-GFP antibody (Fig. 1j,m and Extended Data Fig. 4b). We self-pollinated five of the ZmWAKLY-GFP transgenic events to generate homozygous transgenic lines. All these lines exhibited fewer lesions and significantly reduced GLS scales, but similar morphology and flowering time, compared to B73 (Fig. 1k,l and Supplementary Fig. 3c,d). We backcrossed the remaining five ZmWAKLY-GFP transgenic events to Q11 to generate T1BC1F1 and T1BC3F1 populations. Transgenic plants displayed significantly lower DSI than their nontransgenic siblings (Fig. 1n,o). No obvious difference in GLS resistance between overexpression and native ZmWAKLY alleles suggested that the ZmWAKLY sequence, rather than its expression level, underpinned the variation in GLS resistance. By contrast, plants overexpressing ZmWAKLQ-GFP showed no differences in DSI relative to their nontransgenic siblings (Extended Data Fig. 4c,d).
We generated seven independent transgenic events in B73 overexpressing ZmWAKLC, a chimeric gene encoding a protein consisting of the ZmWAKLY ECD/TM and ZmWAKLQ ICD (Extended Data Fig. 4e). We backcrossed these events to Q11 to produce T1BC2F1 and T1BC3F1 populations. Overall, transgenic plants displayed higher ZmWAKL expression levels and lower DSI values compared to their nontransgenic siblings (Extended Data Fig. 4f,g). The chimeric gene ZmWAKLC lowered DSI in Q11 by 4.5–15.6%, a little less than the ZmWAKLY (7.9–19.5%; Extended Data Fig. 4h), suggesting that the ZmWAKLY ECD has a key role in GLS resistance.
For the other candidate, ZmPR5L, we obtained three overexpression transgenic events and two clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated nuclease 9 (Cas9) knocked out Zmpr5l null mutants. All transgenic plants showed similar disease severity to B73 (Extended Data Fig. 5), suggesting that ZmPR5L is not involved in GLS resistance.
Molecular characterization of ZmWAKL
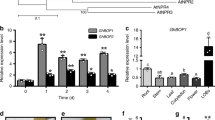
The ZmWAKLQ in NIL-Q11 exhibited higher expression levels than the ZmWAKLY in NIL-Y32 in all tissues except the mesocotyl and root at the seedling stage and the top leaf at the maturity stage (Extended Data Fig. 6a). In plants spray-inoculated at the two-leaf stage in the greenhouse, expression levels of both ZmWAKL alleles were rapidly induced by C. zeina infection, peaking at 6 h postinoculation (hpi), with ZmWAKLY expression being higher than ZmWAKLQ (Extended Data Fig. 6b). In field grown plants infusion-inoculated with inocula into the whorl at the 10-leaf stage, the expression levels of both ZmWAKLY and ZmWAKLQ alleles gradually increased after ~20 dpi and peaked at 29 dpi, with ZmWAKLY expression higher at the peak (Extended Data Fig. 6c).
We transiently transformed onion epidermal cells with constructs ZmWAKLY-EGFP (EGFP, enhanced GFP) and ZmWAKLQ-EGFP for localization studies, finding that both ZmWAKL isoforms localized to the plasma membrane (Fig. 2a and Extended Data Fig. 6d). Using both ZmWAKLY-GFP and ZmWAKLQ-GFP transgenic maize lines, we observed that GFP signal colocalizes with the membrane-impermeable dye FM4-64 (Extended Data Fig. 6e).
a, Subcellular localization of ZmWAKLY-EGFP in onion epidermal cells. Scale bars, 50 μm. b, Split-ubiquitin-based membrane yeast two-hybrid assay indicates that ZmWAKLY could associate with itself. c, SLC assay to detect the self-association of two ZmWAKL isoforms. d, Co-IP assay to detect the self-association of two ZmWAKL isoforms. The immunoprecipitated products were detected by immunoblotting with α-Myc or α-GFP antibody. e,f, Determination of ZmWAKL domains required for self-association in yeast two-hybrid (e) and SLC assays (f). g, Autophosphorylation and kinase activity of the ICDs of two ZmWAKL isoforms. Autoradiography and CBB staining are shown at the top and bottom, respectively. To distinguish the MBP tag from the MBP substrate in the kinase activity assay, we added a superscript ‘a’ to the MBP substrate (MBPa). The experiment was performed three times independently with similar results. h, In vitro phosphorylation of MBPa by ZmWAKLY-GFP and ZmWAKLQ-GFP. ZmWAKLY-GFP and ZmWAKLQ-GFP were separately immunoprecipitated from transgenic plants overexpressing ZmWAKLY-GFP or ZmWAKLQ-GFP. Phosphorylated MBPa was detected by autoradiography (autorad), and the protein loading is shown by CBB staining. ZmWAKL-GFP was detected with α-GFP antibody, with plant actin as a loading control. The Co-IP, SLC assays and phosphorylation assays are repeated at least two times.
Phylogenetic analysis of WAKs/WAKLs reported in rice10,25,26,27, tomato28, Arabidopsis7,29,30,31,32, wheat11,12,33,34 and maize8,9 showed them clustered into three clades with a clear distinction between arginine-aspartate (RD) and non-RD kinases. Both ZmWAKLY and ZmWAKLQ are closer to ZmWAK-RLK1 (ref. 9) than to ZmWAK (ref. 8; Supplementary Fig. 4). We surveyed ZmWAKL sequences in 98 maize inbred lines (Supplementary Table 2) and identified 48 ZmWAKL haplotypes differentiated by numerous SNPs and insertions and deletions (InDels), especially in the extracellular region (Supplementary Fig. 5), which exhibited a nucleotide diversity (π) >3.4-fold higher than the intracellular region (Supplementary Table 3).
The self-association and kinase activity of ZmWAKL
Homodimerization occurs as part of the activation process of some PRRs35. In a split-ubiquitin-based membrane yeast two-hybrid assay, we observed that the GLS-resistant ZmWAKLY, but not the GLS-susceptible ZmWAKLQ, was able to associate with itself (Fig. 2b). We confirmed these results by split-luciferase complementation (SLC) and co-immunoprecipitation (Co-IP) assays (Fig. 2c,d). Furthermore, we determined that the GUB domain in the ZmWAKLY ECD (ZmWAKLY/GUB), but not the STK domain in its ICD (ZmWAKLY/STK), could self-associate in both yeast two-hybrid and SLC assays, while neither ZmWAKLQ/GUB nor ZmWAKLQ/STK was able to do so (Fig. 2e,f).
The maltose-binding protein (MBP)-tagged ZmWAKLY ICD (ZmWAKLY/ICD-MBP), but not its kinase-inactive variant ZmWAKLY/ICD,K391E-MBP, exhibited autophosphorylation and phosphorylated the universal kinase substrate myelin basic protein (MBPa) in vitro, indicating that ZmWAKLY/ICD-MBP has kinase activity (Fig. 2g). By contrast, neither ZmWAKQ/ICD-MBP nor its kinase-inactive variant ZmWAKLQ/ICD,K430E-MBP had kinase activity (Fig. 2g). We also performed a phosphorylation assay by immunoprecipitating ZmWAKLY-GFP and ZmWAKLQ-GFP from their respective overexpressed transgenic plants. We detected kinase activities from immunoprecipitates of both ZmWAKL-GFP isoforms, as indicated by phosphorylated MBPa (Fig. 2h). Given that ZmWAKLQ has no kinase activity in vitro, we speculate that a protein(s) coprecipitated with ZmWAKLQ-GFP may phosphorylate MBPa.
ZmWIK is a coreceptor of ZmWAKL
Plant PRRs usually interact with a coreceptor to recognize the substrate and transduce signal36. To identify a possible ZmWAKL coreceptor, we performed repeated IP using extracts from transgenic plants overexpressing ZmWAKLY-GFP followed by mass spectrometry (MS). Of six identified plasma membrane-tethered kinases (Supplementary Table 4), only a leucine-rich repeat receptor-like serine/threonine-protein kinase (Zm00001d028560, hereinafter referred to as ZmWIK) and a protein kinase (Zm00001d011700, hereinafter referred to as ZmPK2) can interact with both ZmWAKL isoforms (Fig. 3a and Extended Data Fig. 7a–d). ZmPK2, lacking an extracellular receptor-like domain and showing weaker fluorescence and lower gene expression in leaves, is unlikely to be a coreceptor of ZmWAKL (Extended Data Fig. 7a–e).
a, Identification of ZmWAKL and its interacting RLK ZmWIK by IP–MS analysis. b,c, Interaction of ZmWIK with ZmWAKLY or ZmWAKLQ in SLC (b) and Co-IP assay (c). d, Determination of domains responsible for the interactions between ZmWIK and ZmWAKL in a SLC assay. e, Interaction of ZmWIKICD with ZmWAKLY/ICD or ZmWAKLQ/ICD in an in vitro pull-down assay. f, Autophosphorylation and kinase activity of ZmWIKICD. g–i, Reciprocal phosphorylation between ZmWIK and ZmWAKL in vitro. g, Phosphorylation of the kinase-inactive variants ZmWAKLY/ICD,K391E-MBP and ZmWAKLQ/ICD,K430E-MBP by His-ZmWIKICD. h, Phosphorylation of the kinase-inactive variant His-ZmWIKICD,K339E by ZmWAKLY/ICD-MBP or ZmWAKLQ/ICD-MBP. i, Reciprocal phosphorylation between His-ZmWIKICD and ZmWAKLY/ICD-MBP (or ZmWAKLQ/ICD-MBP or their two kinase-inactive variants). j,k, Effects of ZmWAKLY/ICD (or ZmWAKLQ/ICD) on phosphorylation and kinase activity of ZmWIKICD (j), and the quantification of His-ZmWIKICD and MBPa phosphorylation levels (k; n = 3 independent experiments). High, high exposure; Low, low exposure. l, Schematic diagram of ZmWIK and EMS-induced stop codon to generate ZmWIK null mutant. m,n, GLS resistance in the wild-type ZmWIK and ZmWIK null mutant (n = 132). Scale bar, 15 cm. In f–j, protein phosphorylation was detected by autoradiography (upper), and equal protein loading is shown by CBB staining (lower). In k and n, statistical significance was determined by a two-sided Student’s t test. In k, data are shown as means ± s.d. In n, data are displayed as box and whisker plots with individual data points, the dotted cross denotes the mean and the box limits indicate the interquartile range. The Co-IP, SLC, pull-down and phosphorylation assays were repeated at least two times.
The localization of a ZmWIK-GFP fusion in onion epidermal cells and Nicotiana benthamiana leaves was consistent with the plasma membrane location (Extended Data Fig. 7f,g). In a SLC assay, stronger luminescence was seen in the interaction of ZmWIK-nLUC with cLUC-ZmWAKLY than with cLUC-ZmWAKLQ (Fig. 3b). In a Co-IP assay, ZmWIK-Myc could be coprecipitated with ZmWAKLY-GFP and ZmWAKLQ-GFP (Fig. 3c). To identify the domains of ZmWIK and ZmWAKL responsible for the interaction, we divided ZmWIK and ZmWAKL each into two parts, ECD/TM and ICD. In a SLC assay, ZmWIKECD,TM-nLUC interacted with cLUC-ZmWAKLY/ECD,TM, but not with cLUC-ZmWAKLQ/ECD,TM, while ZmWIKICD-nLUC interacted relatively weakly with cLUC-ZmWAKLY/ICD or cLUC-ZmWAKLQ/ICD (Fig. 3d). In a pull-down assay, more His-ZmWAKLY/ICD was pulled down by ZmWIKICD-MBP than His-ZmWAKLQ/ICD (His, histidine; Fig. 3e). To pinpoint the difference between the two ZmWAKLICD isoforms in interacting with ZmWIK, we performed multiple SLC assays. Although cLUC-ZmWAKLY/ICD and cLUC-ZmWAKLQ/ICD showed similar protein abundance, luminescence intensity was significantly higher when ZmWIK-nLUC co-expressed with cLUC-ZmWAKLY/ICD than with cLUC-ZmWAKLQ/ICD (Supplementary Fig. 6). These findings suggest that both the ECD and ICD of ZmWAKLY contribute to its preferential interaction with ZmWIK compared to ZmWAKLQ.
In vitro, the purified His-ZmWIKICD exhibited autophosphorylation and strong transphosphorylation of MBPa, whereas the kinase-inactive variant His-ZmWIKICD,K339E did not (Fig. 3f). Although His-ZmWIKICD had strong kinase activity, it only weakly phosphorylated the two kinase-inactive ZmWAKLICD-MBP variants (Fig. 3g). ZmWAKLY/ICD-MBP also showed autophosphorylation but weak transphosphorylation of His-ZmWIKICD,K339E, while ZmWAKLQ/ICD-MBP displayed neither autophosphorylation nor transphosphorylation (Fig. 3h). We speculate that the use of kinase-inactive variants as substrates may underlie the relatively weak reciprocal phosphorylation, like what has been observed in the previous report37. When ZmWAKLY/ICD-MBP and His-ZmWIKICD were mixed in vitro, they enhanced each other’s phosphorylation levels, with His-ZmWIKICD being more strongly phosphorylated than ZmWAKLY/ICD-MBP. Reduced phosphorylation levels were observed when using ZmWAKLQ/ICD-MBP or two kinase-inactive ZmWAKLICD-MBP variants in place of ZmWAKLY/ICD-MBP (Fig. 3i). His-ZmWIKICD strongly phosphorylated MBPa and adding ZmWAKLY/ICD-MBP further increased the phosphorylation levels of both His-ZmWIKICD and MBPa. Intriguingly, ZmWAKLQ/ICD-MBP had similar effects, although to a lesser extent (Fig. 3j,k).
Two transgenic lines overexpressing ZmWIK showed significantly fewer GLS symptoms compared to B73 (Extended Data Fig. 7h–j). An ethyl methanesulfonate (EMS) induced null mutant Zmwik, harboring a premature translation termination codon, was backcrossed into NIL-Y32. The plants with Zmwik were more susceptible to GLS than wild-type counterparts in the field trials (Fig. 3l–n), suggesting that ZmWIK may act downstream of ZmWAKLY in GLS resistance. Overall, these data indicate that ZmWIK binds to ZmWAKLY at the plasma membrane and lack of either protein reduces GLS resistance. Moreover, they mutually phosphorylate each other, with more frequent phosphorylation made by ZmWAKLY to ZmWIK. We thus speculate that ZmWIK may be a coreceptor of ZmWAKL.
ZmWAKL/ZmWIK interacts with ZmBLK1 for immune signaling
RLCKs, Botrytis-induced kinase 1 (BIK1) and PBL1 (avrPphB susceptible 1-like 1), have emerged as core components linking PRRs to downstream defenses38. We hypothesized that a similar RLCK might contribute to ZmWAKL-mediated immunity. Thus, two maize BIK1/PBL1 homologs, ZmBLK1 (Zm00001d034662) and ZmBLK1-1 (Zm00001d012958), sharing 61.1%/69.0% and 60.4%/69.0% identical sequences to AtBIK1 and AtPBL1, respectively, were identified (Supplementary Fig. 7a). Phylogenetic analysis indicated that ZmBLK1/ZmBLK1-1 clustered in the same clade as OsRLCK176 (refs. 39,40), OsRLCK57 (ref. 41), SlTPK1b (tomato protein kinase 1)42, AtBIK1 (ref. 43) and AtPBL1 (ref. 44), all of which are involved in ROS production via the NADPH oxidase respiratory burst oxidase homolog D (RBOHD). Other RLCKs, OsRLCK185 (ref. 45), AtPBL27 (PBS1-like 27)46, AtPCRK1/2 (PTI compromised RLCK 1/2)47 and AtBSK1 (brassinosteroid-signaling kinase 1)48, are involved in triggering a mitogen-activated protein kinase signaling cascade, clustered in a separate clade (Supplementary Fig. 7b). These findings suggest that ZmBLK1 and ZmBLK1-1 may be involved in ROS-mediated defense responses.
In SLC assays, cLUC-ZmBLK1, but not cLUC-ZmBLK1-1, interacted with ZmWIK-nLUC and ZmWAKLY-nLUC, and to a lesser extent with ZmWAKLQ-nLUC (Fig. 4a and Extended Data Fig. 8a). Using Co-IP assays, we observed that ZmBLK1-Myc was associated with ZmWIK-GFP (Fig. 4b), and specifically with ZmWIKICD-GFP, but not ZmWIKECD,TM-GFP (Fig. 4c). ZmBLK1-Myc was also associated with both ZmWAKL-GFP isoforms in a Co-IP assay (Fig. 4d). ZmBLK1 was reported to contribute to disease resistance and tethered to the plasma membrane through a post-translational N-terminal myristoylation motif49. This plasma membrane localization of ZmBLK1 would facilitate its interaction with ZmWIK and ZmWAKL. With all three proteins present, we detected both ZmBLK1-Myc and cLUC-ZmWIK in the complexes immunoprecipitated by either ZmWAKLY-GFP or ZmWAKLQ-GFP in Co-IP assays (Fig. 4e). Thus, ZmBLK1, ZmWAKL and ZmWIK may form an immune signaling complex at the plasma membrane.
a, Interaction between ZmBLK1 and ZmWIK in an SLC assay. b–d, In Co-IP assay, ZmBLK1 interacts with ZmWIK (b), ZmWIKICD (c) and ZmWAKLY/ZmWAKLQ (d), but not with ZmWIKECD,TM (c). e, Interaction among ZmWIK, ZmBLK1 and ZmWAKLY (or ZmWAKLQ) in Co-IP assay. f, Autophosphorylation and kinase activity of ZmBLK1. g, Reciprocal phosphorylation between ZmBLK1 and ZmWIK. His-ZmWIKICD and ZmBLK1-GST and their kinase-inactive variants His-ZmWIKICD,K339E and ZmBLK1K114E-GST were used for in vitro phosphorylation assays. h,i, Reciprocal phosphorylation between ZmBLK1 and ZmWAKL. The in vitro phosphorylation assays were performed using two ZmWAKLICD-MBP isoforms with the kinase-inactive ZmBLK1K114E-GST (h) and two kinase-inactive ZmWAKLICD-MBP variants with ZmBLK1-GST (i). j, Mutual phosphorylation assays among ZmWAKL, ZmWIK and ZmBLK1. Phosphorylation levels of His-ZmWIKICD,K339E and ZmBLK1K114E-GST (I; n = 2 independent experiments), His-ZmWIKICD and ZmBLK1K114E-GST (II; n = 3 independent experiments), His-ZmWIKICD,K339E and ZmBLK1-GST (III; n = 3 independent experiments) and His-ZmWIKICD and ZmBLK1-GST (IV; n = 4 independent experiments) in the presence of ZmWAKLY or ZmWAKLQ or their kinase-inactive variants. In f–j, autoradiograph (upper) and CBB staining (lower) show phosphorylation and loading, respectively. In j, the data were shown as means ± s.d., and the statistical significance was determined by a paired t test. The Co-IP, SLC and phosphorylation assays were repeated at least two times.
ZmBLK1 is phosphorylated by ZmWAKL/ZmWIK
ZmBLK1-GST (GST, glutathione S-transferase) exhibited autophosphorylation and transphosphorylation of MBPa, but the kinase-inactive variant ZmBLK1K114E-GST did not (Fig. 4f). His-ZmWIKICD had self-phosphorylated and strongly phosphorylated ZmBLK1-GST and, to a lesser extent, ZmBLK1K114E-GST. In contrast, ZmBLK1-GST, although showing significant autophosphorylation, hardly phosphorylated His-ZmWIKICD,K339E (Fig. 4g). With respect to reciprocal phosphorylation between ZmBLK1 and two ZmWAKLICD isoforms, ZmWAKLY/ICD-MBP phosphorylated ZmBLK1K114E-GST, whereas ZmWAKLQ/ICD-MBP didn’t (Fig. 4h). In turn, ZmBLK1-GST phosphorylated ZmWAKLY/ICD,K391E-MBP moderately and ZmWAKLQ/ICD,K430E-MBP more weakly (Fig. 4i). Collectively, these data suggest that reciprocal phosphorylation between the three proteins is not equivalent, with the phosphorelay being primarily directed from ZmWAKLY/ZmWIK to ZmBLK1.
The autophosphorylation and transphosphorylation relationships of ZmWAKL, ZmWIK and ZmBLK1 are likely to be more complex when all three proteins are present. Based on the presumptive phosphorelay pathway, we investigated the phosphorylation level of ZmBLK1 by the ZmWAKL/ZmWIK receptor complex. In the presence of the two kinase-inactive variants His-ZmWIKICD,K339E and ZmBLK1K114E-GST, neither autophosphorylation nor transphosphorylation could be detected for ZmWAKLY/ICD-MBP (Fig. 4j(I)). The phosphorylation of the kinase-inactive variant ZmBLK1K114E-GST by His-ZmWIKICD did not change appreciably upon the addition of ZmWAKLY/ICD-MBP, ZmWAKLQ/ICD-MBP or two kinase-inactive ZmWAKLICD-MBP variants (Fig. 4j (II)). In the presence of the kinase-inactive variant His-ZmWIKICD,K339E, the addition of ZmWAKLY/ICD-MBP, ZmWAKLQ/ICD-MBP or two kinase-inactive ZmWAKLICD-MBP variants did not appear to alter the phosphorylation level of ZmBLK1-GST in any way (Fig. 4j (III)). Notably, His-ZmWIKICD could strongly phosphorylate ZmBLK1-GST, and this transphosphorylation was further increased upon the addition of ZmWAKLY/ICD-MBP, but not by the addition of ZmWAKLQ/ICD-MBP or two kinase-inactive ZmWAKLICD-MBP variants (Fig. 4j (IV)).
In N. benthamiana, we found ZmWAKLY-GFP caused more phosphorylation of ZmBLK1-Myc than ZmWAKLQ-GFP when co-expressed with cLUC-ZmWIK (Extended Data Fig. 8b). The cLUC-ZmWIK and ZmBLK1-Myc constructs were cotransfected into protoplasts isolated from transgenic plants overexpressing ZmWAKLY-GFP or ZmWAKLQ-GFP. In immunoprecipitates obtained using α-Myc magnetic beads, ZmBLK1-Myc was more phosphorylated in the presence of ZmWAKLY-GFP compared to ZmWAKLQ-GFP (Extended Data Fig. 8c). The more ZmBLK1-Myc phosphorylated, the higher its kinase activity (Extended Data Fig. 8d).
We speculate that in the ZmWAKL/ZmWIK/ZmBLK1 complex, ZmWIK directly phosphorylates ZmBLK1, with ZmWAKLY enhancing this, while ZmWAKLQ does not. These findings support an immune phosphorelay from ZmWAKLY to ZmWIK to ZmBLK1 in GLS resistance.
ZmBLK1 interacts with and phosphorylates ZmRBOH4
In Arabidopsis, BIK1 directly binds to and phosphorylates the N-terminal domain of RBOHD, leading to a ROS burst that mediates plant immunity43,50. In maize genome, six RBOHs were identified, of which ZmRBOH4 was the most closely related to AtRBOHD with 63.3% sequence identity (Extended Data Fig. 9a). In SLC assays, ZmRBOH4 interacted with ZmBLK1 but not ZmBLK1-1; furthermore, the N-terminal domain of ZmRBOH4 directly interacted with ZmBLK1, but not with two ZmWAKL isoforms or ZmWIK (Fig. 5a and Extended Data Fig. 9b,c). We confirmed these results by pull-down assays (Fig. 5b and Extended Data Fig. 9d). For the other five ZmRBOHs, ZmRBOH1/ZmRBOH5/ZmRBOH6 but not ZmRBOH2/ZmRBOH3 interacted with ZmBLK1 in SLC assays (Extended Data Fig. 9e). However, unlike ZmRBOH4, which has high and pathogen-inducible gene expression in leaves (Extended Data Fig. 9f), ZmRBOH1 showed relatively low gene expression, which could not be induced by pathogen inoculation, and ZmRBOH5/ZmRBOH6 were exclusively expressed in the anthers (Supplementary Fig. 8a,b). Hence, only ZmRBOH4 is likely involved in the immune signaling against GLS.
a, N-ZmRBOH4 interacts with ZmBLK1 in SLC assay. b, In vitro MBP pull-down assay to show the interaction of His-ZmBLK1 with N-ZmRBOH4-MBP. c, ZmWAKL does not phosphorylate N-ZmRBOH4 in an in vitro kinase assay. d, ZmWIK weakly phosphorylates N-ZmRBOH4 in vitro. e, ZmBLK1 strongly phosphorylates N-ZmRBOH4 in vitro. f, Phosphorylation levels of ZmWAKLICD, ZmWIKICD, ZmBLK1 and N-ZmRBOH4 when mixed in vitro. ZmWAKLY/ICD-MBP, ZmWAKLQ/ICD-MBP and their corresponding kinase-inactive variants were separately added to a mixture of His-ZmWIKICD, ZmBLK1-GST and His-N-ZmRBOH4 to detect phosphorylation levels. g, Effects of the kinase-inactive variants His-ZmWIKICD,K339E and/or ZmBLK1K114E-GST on phosphorylation of N-ZmRBOH4. His-ZmWIKICD/ZmBLK1-GST or His-ZmWIKICD,K339E/ZmBLK1-GST or His-ZmWIKICD/ZmBLK1K114E-GST or His-ZmWIKICD,K339E/ZmBLK1K114E-GST were separately added to a mixture of ZmWAKLY/ICD-MBP and His-N-ZmRBOH4. h,i, Representative images of ear leaves (h) and GLS scale values (i) of B73 and ZmRBOH4 knockout plants at 44 dpi with C. zeina in the field. Each value represents the disease scale from one plant. The number of plants is indicated below. Scale bar, 15 cm. In c–g, protein phosphorylation was detected by autoradiography (upper), and equal protein loading is shown by CBB staining (lower). In i, data are shown as violin plots with individual data points; different lowercase letters indicate a significant difference (P < 0.05) based on one-way ANOVA with the LSD test. The SLC, pull-down and phosphorylation assays were repeated at least two times.
Both ZmWAKLY/ICD-MBP and His-ZmWIKICD showed autophosphorylation, but phosphorylated His-N-ZmRBOH4 not at all or weakly, respectively (Fig. 5c,d). This is consistent with the lack of direct interaction between ZmRBOH4 and ZmWAKLY (or ZmWIK; Extended Data Fig. 9b–d). By contrast, ZmBLK1-GST strongly phosphorylated His-N-ZmRBOH4 (Fig. 5e). When all four proteins, ZmWAKL, ZmWIK, ZmBLK1 and ZmRBOH4, were mixed in vitro, their phosphorylation levels varied substantially, from strongly phosphorylated ZmBLK1-GST and His-N-ZmRBOH4 to barely phosphorylated His-ZmWIKICD to undetectably phosphorylated ZmWAKLY/ICD-MBP. Phosphorylation of His-N-ZmRBOH4 was somewhat enhanced in the presence of ZmWAKLY/ICD-MBP compared to ZmWAKLQ/ICD-MBP and the two kinase-inactive ZmWAKLICD-MBP variants (Fig. 5f). In the presence of kinase-active ZmWAKLY/ICD-MBP and ZmBLK1-GST, His-N-ZmRBOH4 displayed weaker phosphorylation when His-ZmWIKICD was replaced by its kinase-inactive variant His-ZmWIKICD,K339E. However, the substitution of ZmBLK1-GST by its kinase-inactive variant ZmBLK1K114E-GST almost eliminated His-N-ZmRBOH4 phosphorylation. When both kinase-inactive His-ZmWIKICD,K339E and ZmBLK1K114E-GST variants were present, no His-N-ZmRBOH4 phosphorylation could be detected (Fig. 5g). We interpreted these results as evidence that ZmBLK1 is the major upstream kinase phosphorylating N-ZmRBOH4-GST in this assay, and ZmRBOH4 may join the ZmWAKLY/ZmWIK/ZmBLK1 complex to form an immune module.
To verify the function of ZmRBOH4, we knocked out ZmRBOH4 in B73 using CRISPR/Cas9. Notably, these two knocked out lines exhibited significantly reduced plant height and increased GLS susceptibility compared with wild-type plants under artificial inoculation (Fig. 5h,i and Extended Data Fig. 10), indicating ZmRBOH4 is involved in regulating GLS resistance.
ZmWAKL-mediated immunity against GLS in vivo
As noted above, ZmWAKL could be induced by C. zeina infection with a peak at 6 hpi (Extended Data Fig. 6b). Here we wanted to know what would happen to ZmWAKLY and ZmWAKLQ proteins in response to C. zeina infection. Transgenic plants overexpressing ZmWAKLY-GFP inoculated with C. zeina showed increased levels of phosphorylated ZmWAKLY, peaking at 6 hpi, with stronger kinase activity (Fig. 6a). However, in a parallel experiment, ZmWAKLQ-GFP appeared to be unaffected by C. zeina infection, exhibiting only basal phosphorylation level and kinase activity (Fig. 6b). Like what was previously observed (Fig. 2h), we speculate that coprecipitated protein(s) in the ZmWAKLQ-GFP immunoprecipitate, rather than ZmWAKLQ-GFP itself, may be responsible for this basal activity. To gain insight into the phosphorylation changes of the other proteins, we performed proteomic and phosphoproteomic analyses on transgenic plants overexpressing ZmWAKLY-GFP and ZmWAKLQ-GFP collected at 6 hpi. We detected that the phosphorylation levels of ZmBLK1 and ZmRBOH4 showed significant increases in ZmWAKLY-GFP plants compared to those in ZmWAKLQ-GFP plants (Fig. 6c and Supplementary Table 5).
a,b, Phosphorylation and kinase activity of ZmWAKL in plants overexpressing ZmWAKLY-GFP (a) or ZmWAKLQ-GFP (b) after C. zeina infection. The phosphorylated ZmWAKL was detected with an α-phospho-(Ser/Thr) antibody; phosphorylation was detected by autoradiography (autorad) and protein loading is shown by CBB staining. These assays were repeated two times with the same results. c, Protein phosphorylation intensity of ZmBLK1 and ZmRBOH4 at 6 h after C. zeina inoculation in phosphoproteomic analyses (n = 3). d–f, C. zeina-induced ROS burst in NILs (d, n = 16), ZmWIK mutants (e, n = 16) and ZmRBOH4 knockout plants (f, n = 21). The isolated leaves (V4 stage) were used to examine C. zeina-induced ROS, recorded as relative luminescence units (RLUs). g, Working model for ZmWAKL-mediated GLS resistance. PM, plasma membrane. Scale bar, 15 cm. In c, the violin plots show the protein phosphorylation intensity of ZmBLK1 and ZmRBOH4. The thin dashed lines indicate the interquartile range. The thick dashed line shows the medians. Statistical significance was determined by a paired t test. In d–f, data are means ± s.e. These assays were repeated three times with similar results.
Considering our evidence that immune signaling, initiated by ZmWAKLY in response to C. zeina attack, eventually converges to ZmRBOH4 via ZmWIK and ZmBLK1, it appears that GLS resistance may be associated with a ROS burst. We observed that NIL-Y32 exhibited higher C. zeina-induced ROS levels compared to NIL-Q11 (Fig. 6d) and Zmwik and Zmrboh4 null mutants showed lower C. zeina-induced ROS levels than wild-type plants (Fig. 6e,f).
Working model of maize WAKL–WIK–BLK1–RBOH4 immune module
We characterized the role of the cell-wall-associated RLK ZmWAKL in QDR to GLS in maize. Our investigations suggest the following model: ZmWAKLY could self-associate and interact with the coreceptor ZmWIK to form the ZmWAKLY/ZmWIK receptor complex at the plasma membrane. Upon challenge by the pathogen C. zeina, ZmWAKLY is activated and transiently increases its phosphorylation activity, and together with ZmWIK, transmits immune signals to ZmBLK1 and then to ZmRBOH4, which triggers a ROS burst to incur innate immunity (Fig. 6g). In contrast to ZmWAKLY, ZmWAKLQ is unable to associate with itself, shows no autophosphorylation or kinase activity and interacts weakly with ZmWIK. Upon pathogen challenge, ZmWAKLQ shows no detectable increase in its phosphorylation activity, thus failing to trigger immune responses, leading to GLS susceptibility (Fig. 6g). The maize ZmWAKL–ZmWIK–ZmBLK1–ZmRBOH4 immune module perceives pathogen invasion, transduces immune signals and activates defense responses, eventually conferring quantitative resistance to GLS.
Discussion
Identifying the causative gene underlying QDR is challenging due to the difficulties with the genetic dissection and verification of this quantitative trait. Here we adopted a sequential fine-mapping strategy and multiple transgenic approaches to demonstrate that ZmWAKL is the causative gene for qRgls1 (Fig. 1). Characterizing ZmWAKL allowed us to identify its coreceptor ZmWIK, a plasma-membrane-localized RLK (Figs. 2 and 3). Thereafter, partly based on analogies to systems found in Arabidopsis, we identified the ZmBLK1 and ZmRBOH4 as downstream components of the ZmWAKLY/ZmWIK receptor complex (Figs. 4 and 5).
Extracellular ROS are produced primarily through activation of RBOHs at the plasma membrane and act as a pivotal early component of PTI responses51. Besides, RBOHs are crucial for growth and development51,52, like knocking out ZmRBOH4 results in weaker plants (Extended Data Fig. 10). The reduced vitality of ZmRBOH4 mutants might also contribute to the increased GLS susceptibility (Fig. 5h,i).
In cereal crops, the molecular mechanism of ROS production and its contribution to QDR are not completely understood. Therefore, the discovery of the maize ZmWAKL–ZmWIK–ZmBLK1–ZmRBOH4 immune module will have a significant influence on the conceptual underpinnings of QDR in plants.
Methods
Plant materials
In our previous study, the major QTL-qRgls1 is restricted to a ~1.4-Mb region flanked by makers GZ204 and IDP5 (ref. 22). To fine-map qRgls1, the recombinants were self-pollinated and backcrossed to Q11 to develop BC2F5 (1,865 plants), BC5F7 (1,055 plants) and BC7F7 (1,162 plants) populations. The five BC7F7 recombinants were subsequently backcrossed to Q11 to generate BC8F7 generations, which were then self-pollinated to develop BC8F8 homozygous lines with/without Y32 segments. Comparison of Y32 donor regions with GLS resistance allowed us to confirm the final qRgls1 region. From the recombinant IV, we obtained a pair of near-isogenic lines differing at qRgls1, namely NIL-Y32 (with qRgls1) and NIL-Q11 (without qRgls1). The two NILs had 96.12% genetic identity (as assessed with the Axiom_M6H60k genotyping chip, Thermo Fisher Scientific) and were used for exploring the molecular mechanism underlying ZmWAKL-mediated innate immunity. In addition, a collection of 98 maize inbred lines (Supplementary Table 2) was used for examining allelic variations of the ZmWAKL locus.
The EMS mutant line Zmwik was obtained from the Maize EMS-induced Mutant Database (http://www.elabcaas.cn/memd/public/index.html#/; mutant ID: EMS4-0073f9). The mutant was generated in the B73 background and contained the heterozygous ZmWIK locus. The mutant was backcrossed twice to NIL-Y32, followed by self-pollination, to introduce ZmWAKLY into the ZmWIK wild-type and its homozygous Zmwik mutant to evaluate GLS resistance.
Field assays
The fine-mapping populations, maize inbred lines and ZmWIK mutants were planted in Baoshan (Yunnan, China) and naturally infected with the pathogenic fungus C. zeina. Baoshan is the ideal place for GLS development due to sufficient natural disease stress. The seeds were sown at 5 m in length with 0.5 m between rows and 0.25 m between plants within a row.
In Beijing, transgenic plants and NILs were planted in the same way as in Baoshan, except for using the 4-m long plot and being artificially inoculated with C. zeina. The GLS inoculum was prepared using the pathogenic fungus C. zeina. The fungus was cultured on potato dextrose agar medium, and the spores were washed off with ddH2O to prepare a spore inoculum at a concentration of 5 × 103 spores per ml. Plants were inoculated at the 10-leaf stage by pouring 5-ml inoculum into the leaf whorl. Samples were collected at different time points after inoculation, as specified in each experiment. Each sample contains leaf tissues collected from at least three individual plants. For gene expression analysis, one leaf punch per plant was collected from the middle of the inoculated leaf, and each genotype/treatment sample contained three punches, which were pooled and stored at −80 °C. For scanning electron microscope (SEM) analysis, four punches across the center of each leaf were collected.
The GLS symptoms are scored three times at 1-week intervals, starting 2 weeks after pollination. The number and size of lesions on the leaves of the entire plant were used to assess the GLS severity. GLS scale was rated using the following scale: 1 (highly resistant), 3 (resistant), 5 (intermediate resistant/susceptible), 7 (susceptible) and 9 (highly susceptible). To convert the scale to a DSI, scales 1, 3, 5, 7 to 9 were assigned to a distinct numerical value of 0, 0.25, 0.5, 0.75 and 1, respectively.
Days to heading, silking and anthesis were recorded for each plant from NILs and homozygous transgenic lines.
Sequential fine-mapping of qRgls1
Newly developed markers in the qRgls1 region were used to genotype recombinants (Supplementary Table 1). In each generation, new recombinants within the mapped qRgls1 region were selected and backcrossed to Q11 to produce progeny for fine-mapping. The qRgls1 locus was fine-mapped by comparing the genotypes of the recombinants with their deduced phenotypes based on the resistant performance of their progeny. A significant difference in the DSI between the heterozygous and homozygous genotypes of the offspring indicates the presence of the resistance allele at qRgls1 in the heterozygous region of their parental recombinant; otherwise, there is no resistance allele at qRgls1.
Genetic transformation
From the positive Y32 BAC clone 57-9-1-93, a 9.2 kb genomic fragment, containing the 2.1-kb promoter, 2.2-kb ZmWAKLY coding and 4.9-kb 3′-UTRs, was subcloned into pCAMBIA3301 according to the method described previously8. To construct overexpression vector, the fused gene of ZmWAKL coding sequence (CDS) and EGFP was cloned into the express vector pBCXUN-Myc driven by the maize ubiquitin promoter as described earlier53. In addition, we constructed overexpression vectors for the chimeric genes ZmWAKLC, ZmPR5L and ZmWIK. To knockout the endogenous genes ZmPR5L and ZmRBOH4, CRISPR/Cas9 vectors were constructed following the protocol described previously54. These vectors were transferred to Agrobacterium strain EHA105 and then transformed into the maize inbred line B73.
The genotypes were confirmed by PCR or sequencing standard PCR products. All T1-positive or edited plants from each transgenic event were self-pollinated to produce homozygous transgenic plants or backcrossed to the susceptible parent Q11 or NIL-Y32 to produce backcross populations for functional verification.
Plant protein extraction and immunoblot analysis
Plant total protein was extracted from maize or tobacco leaves. The tissue sample was ground in liquid nitrogen and resuspended in an equal volume (1:1 fresh wt/vol) of the extraction buffer (50 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 mM dithiothreitol (DTT), 1 mM phenylmethylsulfonyl fluoride (PMSF) and 0.2 % Triton X-100) on ice for at least 30 min and then centrifuged at 12,000g for 30 min at 4 °C to obtain the supernatant. The remaining supernatant was incubated with ~5 μl magnetic beads for 2 h at 4 °C. The beads were magnetically separated, washed twice and then heated in 100 μl of 1× SDS sample buffer at 95 °C for 5 min. The protein sample was subjected to electrophoresis on a 10% (wt/vol) polyacrylamide gel and transferred to a polyvinylidene fluoride blotting membrane (GE HealthCare, A10116498). The membrane was incubated for 1 h at room temperature with 5% nonfat milk in tris-buffered saline (20 mM Tris–HCl (pH 7.6) and 150 mM NaCl) containing 0.05% Tween 20 (TBST). All antibody incubations were performed in 1× phosphate-buffered saline (1× PBS; 137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.4 mM KH2PO4, pH 7.4). The fused protein was detected with the corresponding tag antibody at a dilution of 1:2,000 (vol/vol) overnight at 4 °C. α-Actin (dilution of 1:2,000 (vol/vol)) was used as an endogenous control (EASYBIO, BE0028). The membrane was incubated for 1 h with an immunoglobulin horseradish peroxidase-conjugated secondary antibody at a dilution of 1:5,000 (vol/vol) to visualize the signal.
Subcellular localization
The CDS of ZmWAKLY, ZmWAKLQ and ZmWIK were inserted into the vector pEZS-NL to create EGFP-fused expression constructs driven by the 35S promoter. Similarly, the ZmWIK CDS was cloned into pSuper1300-GFP to generate the fusion construct. The ZmWIK-GFP constructs were transformed into tobacco leaves using Agrobacterium-mediated method. The ZmWAKLY-EGFP, ZmWAKLQ-EGFP and ZmWIK-EGFP constructs were also mixed with nano-grade gold particles and bombarded into onion epidermal cells, which were then cultured at 28 °C in the dark for 8–10 h. The GFP signals were visualized under a confocal microscope (Zeiss). Plasmolysis was achieved by incubating onion epidermal cells in 0.3 g ml−1 sucrose for 5 min.
Fluorescence was examined in the root epidermal tissues of 1-week-old ZmWAKLY-GFP or ZmWAKLQ-GFP overexpression transgenic plants and B73 under a confocal microscope. The membrane-impermeable dye FM4-64 (MedchemExpress, HY-103466) was used as the plant membrane marker.
Phylogenetic analysis
The amino acid sequences of reported immune-related WAKs/WAKLs and RLCKs were downloaded from the National Center for Biotechnology Information (NCBI) database (https://www.ncbi.nlm.nih.gov/). The ZmRBOH1-6 amino acid sequences were retrieved from the Gramene database (http://www.gramene.org/). The full-length sequences were used for the phylogenetic analysis, and the phylogenetic trees were constructed using the neighbor-joining method in MEGA 7.0 (http://www.megasoftware.net). Bootstrap values from 1,000 pseudo-replicates were used to provide support for the nodes in the phylogenetic tree. The conserved domains were predicted using the NCBI Conserved Domains Database (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi).
Yeast two-hybrid assay
The DUAL membrane system was used to verify the self-association of ZmWAKL. The signal peptide sequences of ZmWAKLY and ZmWAKLQ were removed, and the remaining sequences were cloned into the pBT3-SUC vector. The full-length CDS of ZmWAKLY and ZmWAKLQ were cloned into the pPR3-N vector. These vectors were cotransformed into the yeast strain NMY51 to detect protein–protein interactions according to the manufacturer’s user guide (Clontech). Then, the GUB domain (amino acids 46–155 in ZmWAKLY and amino acids 37–182 in ZmWAKLQ) and the intracellular kinase domain (amino acids 362–630 in ZmWAKLY and amino acids 404–666 in ZmWAKLQ) were cloned into pGADT7 and pGBKT7 vectors, respectively, to verify the interaction domains. These vectors were cotransformed into the Y2HGold strain, and the protein interactions were tested on a selective medium.
SLC assay
The CDS of ZmWAKLY, ZmWAKLQ, ZmBLK1, ZmWIK, ZmRBOH4 and their gene segments were cloned into the JW771-35S-CLuc vector (cLUC) and JW772-35S-NLuc vector (nLUC), respectively, to generate fusion proteins with the C-terminal or N-terminal fragment of the luciferase gene. ZmWIK and ZmWAKL were divided into their ECD/TM and ICD, marked as ZmWIKECD,TM (1-269 aa) and ZmWIKICD (270-594 aa), ZmWAKLY/ECD,TM (1-324 aa) and ZmWAKLY/ICD (325-665 aa), ZmWAKLQ/ECD,TM (1-363 aa) and ZmWAKLQ/ICD (364-704 aa). The N-terminal domain was marked as N-ZmRBOH4 (1-377 aa). The pairs of constructs were co-infiltrated into N. benthamiana leaves using the previously reported method55. Two days after inoculation, 1 mM luciferin (Promega, E1601) was sprayed onto the inoculated leaves, and the luminescence signal was measured using the Chemiluminescent Imaging System (Tanon).
The luminescence signals were analyzed using ImageJ Launcher software (National Institutes of Health) to determine their intensities, so-called the mean gray value. This assay was immediately followed by protein extraction and immunoblotting with α-LUC antibody (Abcam, ab181640), and the immunoblot bands were measured using ImageJ Launcher as well. Total protein of the infiltrated leaf tissues was extracted and detected as described above.
Co-IP assay
To generate ZmWAKLY-Myc, ZmWAKLY-GFP, ZmWAKLQ-Myc, ZmWAKLQ-GFP, ZmWIK-Myc, ZmWIKECD/TM-GFP, ZmWIKICD-GFP and ZmBLK1-Myc, the CDS or gene segments for ZmWAKL, ZmWIK and ZmBLK1 were amplified and then cloned into pSuper1300 (Myc-tag or GFP-tag). The resulting plasmids were transformed into N. benthamiana leaves and expressed for about 48 h. Total protein from the infiltrated leaf tissues was extracted as described above, and the supernatant was incubated with the α-GFP magnetic beads (MBL, D153-11) at 4 °C for 2 h. The products were analyzed and detected by immunoblotting with α-Myc antibody (ABclonal, AE010) and α-LUC antibody (Abcam, ab181640). GFP and GFP-fusion proteins were detected by immunoblotting with α-GFP antibody (ABclonal, AE012).
IP–MS
The immunocomplexes were analyzed by mass spectrometry (MS) at the China Agricultural University Mass Spectrum Laboratory. Briefly, ~2 g leaf of Ubipro:ZmWAKLY-GFP transgenic plants were collected and ground in a mortar using liquid nitrogen. The GFP-tagged fusion protein complex was extracted as described above and separated by sodium dodecyl-sulfate polyacrylamide gel electrophoresis (SDS–PAGE). The gel lanes were cut, sliced and subjected to in-gel digestion with trypsin. After destaining and tryptic digestion, peptides were extracted and redissolved in 25 μl (0.1%) trifluoroacetic acid. In total, 6 μl of extracted peptides were analyzed by LTQ Orbitrap Velos mass spectrometer (Thermo Fisher Scientific). The Mascot search engine Mascot Server 2.3 (Matrix Science) was used for protein identification by searching against the UniProt protein database (https://www.uniprot.org/). The false discovery rate (FDR) was also set to 0.01 for protein identifications. The significance threshold was set at P < 0.05, and a minimum number of significant unique sequences was set to 1.
In vitro pull-down assay
In vitro pull-down assay was carried out as previously described56. In brief, 0.5 µg of His-ZmWAKLY/ICD or His-ZmWAKLQ/ICD or His-TF-ZmWAKLY/ICD or His-TF-ZmWAKLQ/ICD or His-ZmWIKICD or His-ZmBLK1 protein was incubated with 5 µg of ZmWIKICD-MBP or N-ZmRBOH4-GST or N-ZmRBOH4-MBP and immunoprecipitated by MBP or GST agarose resin at 4 °C for 2 h. The mixture was gathered by centrifugation at 500g for 5 min, followed by washing with phosphate-buffered saline (PBS) buffer five times. Proteins were separated by 10% (wt/vol) SDS–PAGE and detected with α-MBP antibody (ABclonal, AE016), α-GST antibody (Yeasen, 30903ES10) and α-His antibody (Yeasen, 30404ES60).
In vitro phosphorylation assay
ZmWAKLY/ICD-MBP, ZmWAKLQ/ICD-MBP, His-ZmWIKICD, ZmBLK1-GST and their corresponding kinase-inactive variants, His-N-ZmRBOH4 fusion protein and MBPa (EMB Millipore, 13-104) were used for in vitro phosphorylation assay in the presence of (32P)-γ-ATP.
For the in vitro phosphorylation assay, the recombinant proteins were incubated in a kinase buffer (20 mM Tris–HCl (pH 7.5), 10 mM MgCl2, 1 mM CaCl2 and 1 mM DTT) in the presence of 1 mM unlabeled ATP and 1 µCi of (32P)-γ-ATP for 30 min at 30 °C. The reactions were stopped by adding 5× SDS loading buffer (GenStar, E153-10). Proteins were separated by SDS–PAGE, followed by staining with CBB overnight with decolorization. The phosphorylation status of the fusion proteins was analyzed by autoradiography using a Typhoon 9410 Variable Mode Imager (GE HealthCare).
In vivo phosphorylation assay
ZmBLK1 protein phosphorylated in vivo was detected using α-phospho-(Ser/Thr) antibody (Abcam, ab117253). Briefly, proteins were extracted from the protoplasts of Ubipro:ZmWAKLY-GFP and Ubipro:ZmWAKLQ-GFP transgenic plants co-expressing cLUC-ZmWIK and ZmBLK1-Myc. Proteins were also extracted from the N. benthamiana leaves co-expressing cLUC-ZmWIK, ZmBLK1-Myc and ZmWAKL-GFP or GFP. The ZmWAKL protein phosphorylation level was identified by western blotting with α-phospho-(Ser/Thr) antibody (Abcam, ab117253). The proteins were extracted from the samples collected at different times after C. zeina infection. Protein extraction and detection were consistent with the methods described above.
Sample preparation for phosphoproteomic and proteomic
The sample was first grinded with liquid nitrogen, then the powder was added to four volumes of lysis buffer (including 10 mM dithiothreitol, and 1% protease inhibitor cocktail, 50 μM PR-619, 3 μM tryptic soy agar (TSA), 50 mM N-acetylmuramic acid (NAM) and 1% phosphatase inhibitor), ultrasonic cracking. An equal volume of Tris-saturated phenol (pH 8.0) was added and centrifugated at 5,500g at 4 °C for 10 min. Then the supernatant was collected and five times the volume of 0.1 M ammonium acetate/methanol was added to precipitate overnight. The precipitates were washed with methanol and acetone, respectively. Finally, the precipitate was redissolved in 8 M urea, and the protein concentration was determined with bicinchoninic acid (BCA) kit according to the manufacturer’s instructions. All samples were labeled with TMT 6-plex reagent (Thermo Fisher Scientific, 90068) according to the manufacturer’s instructions. The labeled samples were combined and dried for further analysis.
Identification of peptides and phosphopeptides
The mixed TMT 6-labeled samples were analyzed by high pH reverse phase high-performance liquid chromatography (HPLC) with Agilent 300Extend C18 as the chromatographic column. The peptide segments were separated using a gradient ranging from 8% to 32% acetonitrile (pH 9.6) within 60 min. The separated peptide segments were combined into eight components. The combined components were subjected to vacuum freeze-drying, and subsequent operations were carried out. Peptide mixtures were first incubated with IMAC microspheres suspension with vibration in loading buffer (50% acetonitrile/0.5% acetic acid). To remove the nonspecifically adsorbed peptides, the immobilised metal affinity chromatography (IMAC) microspheres were washed with 50% acetonitrile/0.5% acetic acid and 30% acetonitrile/0.1% trifluoroacetic acid, sequentially. To elute the enriched phosphopeptides, an elution buffer containing 10% NH4OH was added and the enriched phosphopeptides were eluted with vibration. The supernatant containing phosphopeptides was collected and lyophilized for liquid chromatography with tandem mass spectrometry (LC–MS/MS) analysis. All raw data were retrieved using Proteome Discoverer (v2.4.1.15). Database is Zea_ mays_ 4577_ PR_ 20221123 (6,3235 sequences). A reverse library was added to the database to calculate the false positive rate (FPR) caused by random matching, and common contaminated libraries were added to the database to eliminate the impact of contaminated proteins in identification results. The quantitative method is set to TMT 6-plex, and the FPR for protein, peptide and peptide-spectrum match (PSM) identification is set to 1%.
Oxidative burst assay
To determine pathogen-induced ROS accumulation, the GLS pathogen C. zeina was used to activate the innate immune system. C. zeina was cultured on the corn leaf powder medium for 1–2 weeks. Then, everything on the surface of the medium, including spores and hyphae, was eluted with sterile water, and the eluted solution’s OD600 was adjusted to 2. The oxidative burst assay was carried out as previously described with slight modifications21. The fourth fully expanded leaves were sliced into 3 mm strips and incubated in 200 µl 1% DMSO overnight. In total, 5 µl C. zeina was added and treated for 2 h. Then 1% DMSO was replaced by 100 µl 2× L-012 (Wako, 120-04891) in 0.05% Silwet L-77, and the strip samples were infiltrated for 1 h. Then 100 µl buffer containing 40 µg ml−1 horseradish peroxidase (Sigma-Aldrich, V900503) was added into the reactions. The luminescence was recorded every 60 s for 1.25 h using the GLOMAX96 Luminometer (Promega).
Inclusion and ethics
This study’s data exclusively comes from corn, without involving any animal experiments. The transgenic planting and artificial inoculation processes are subject to strict regulation. All experimental data are included in the Data availability section.
Statistics analysis
P values and sample sizes (n) are indicated in individual figures and figure legends. Statistical analysis was performed by IBM SPSS Statistics SV26. Statistical differences between the two groups were analyzed by two-sided Student’s t test or paired t test. Statistical significance between more than two groups was analyzed based on one-way analysis of variance (ANOVA) with Tukey’s test or Fisher’s least significant difference (LSD) test. Different lowercase letters indicate a significant difference (P < 0.05).
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
The authors declare that the data supporting the findings of this study are available within the paper and its Supplementary Information files. The reported WAKs/WAKLs and RLCKs’ protein sequences are downloaded from the NCBI (http://www.ncbi.nlm.nih.gov/). The protein sequences of AtRBOHs are downloaded from the Arabidopsis Information Resource database (TAIR, https://www.arabidopsis.org/) and the protein sequences of ZmRBOHs are obtained from the Gramene database (https://www.gramene.org/). The expression data of ZmRBOHs and RLKs are obtained from the Plant Public RNA-seq database (http://ipf.sustech.edu.cn/pub/plantrna/). The B73 genomic sequences in the mapped qRgls1 region are collected from the MaizeGDB (https://www.maizegdb.org/). The sequences of two BAC clones (17-37-1-53 and 57-9-1-93) are available at GenBank accessions OQ435908 and OQ435909, respectively. The genomic sequences of ZmWAKL used for haplotype analysis are available at GenBank under accessions OQ425304–OQ425401. The coding sequences of ZmWAKLY and ZmWAKLQ are available at GenBank accessions OQ421108 and OQ421109, respectively. The genomic and coding sequences of ZmPR5Y and ZmPR5Q are available at GenBank accessions OQ421106–OQ421107 and OQ421110–OQ421111, respectively. The coding sequence of ZmWIK is available at GenBank accession OQ421112. The coding sequences of ZmBLK1 and ZmBLK1-1 are available at GenBank accessions OQ421113 and OQ421114, respectively. The N-terminal of ZmRBOH4 is available at GenBank accession OQ421115. Source data are provided with this paper.
Code availability
All software used in this study is publicly available on the Internet as described in the Methods and Reporting summary.
References
Jones, J. D. & Dangl, J. L. The plant immune system. Nature 444, 323–329 (2006).
Takeuchi, O. & Akira, S. Pattern recognition receptors and inflammation. Cell 140, 805–820 (2010).
Couto, D. & Zipfel, C. Regulation of pattern recognition receptor signalling in plants. Nat. Rev. Immunol. 16, 537–552 (2016).
Wang, W., Feng, B., Zhou, J. M. & Tang, D. Plant immune signaling: advancing on two frontiers. J. Integr. Plant Biol. 62, 2–24 (2020).
Wang, R., He, F., Ning, Y. & Wang, G. L. Fine-tuning of RBOH-mediated ROS signaling in plant immunity. Trends Plant Sci. 25, 1060–1062 (2020).
DeFalco, T. A. & Zipfel, C. Molecular mechanisms of early plant pattern-triggered immune signaling. Mol. Cell 81, 3449–3467 (2021).
Diener, A. C. & Ausubel, F. M. Resistance to Fusarium oxysporum 1, a dominant Arabidopsis disease-resistance gene, is not race specific. Genetics 171, 305–321 (2005).
Zuo, W. et al. A maize wall-associated kinase confers quantitative resistance to head smut. Nat. Genet. 47, 151–157 (2015).
Hurni, S. et al. The maize disease resistance gene Htn1 against northern corn leaf blight encodes a wall-associated receptor-like kinase. Proc. Natl Acad. Sci. USA 112, 8780–8785 (2015).
Hu, K. et al. Improvement of multiple agronomic traits by a disease resistance gene via cell wall reinforcement. Nat. Plants 3, 17009 (2017).
Saintenac, C. et al. Wheat receptor-kinase-like protein Stb6 controls gene-for-gene resistance to fungal pathogen Zymoseptoria tritici. Nat. Genet. 50, 368–374 (2018).
Shi, G. et al. The hijacking of a receptor kinase-driven pathway by a wheat fungal pathogen leads to disease. Sci. Adv. 2, e1600822 (2016).
Crous, P. W. et al. Species of Cercospora associated with grey leaf spot of maize. Stud. Mycol. 55, 189–197 (2006).
Kuki, M. C. et al. Genome wide association study for gray leaf spot resistance in tropical maize core. PLoS ONE 13, e0199539 (2018).
Mueller, D. S. et al. Corn yield loss estimates due to diseases in the United States and Ontario, Canada, from 2016 to 2019. Plant Health Prog. 21, 238–247 (2020).
Benson, J. M., Poland, J. A., Benson, B. M., Stromberg, E. L. & Nelson, R. J. Resistance to gray leaf spot of maize: genetic architecture and mechanisms elucidated through nested association mapping and near-isogenic line analysis. PLoS Genet. 11, e1005045 (2015).
Sun, H., Zhai, L., Teng, F., Li, Z. & Zhang, Z. qRgls1.06, a major QTL conferring resistance to gray leaf spot disease in maize. Crop J. 9, 342–350 (2020).
Du, L. et al. Genetic mapping of quantitative trait loci and a major locus for resistance to grey leaf spot in maize. Theor. Appl. Genet. 133, 2521–2533 (2020).
Zhang, X., Yang, Q., Rucker, E., Thomason, W. & Balint-Kurti, P. Fine mapping of a quantitative resistance gene for gray leaf spot of maize (Zea mays L.) derived from teosinte (Z. mays ssp. parviglumis). Theor. Appl. Genet. 130, 1285–1295 (2017).
Yang, Q. et al. A gene encoding maize caffeoyl-CoA O-methyltransferase confers quantitative resistance to multiple pathogens. Nat. Genet. 49, 1364–1372 (2017).
Wang, H. et al. A teosinte-derived allele of a MYB transcription repressor confers multiple disease resistance in maize. Mol. Plant 14, 1846–1863 (2021).
Zhang, Y. et al. QTL mapping of resistance to gray leaf spot in maize. Theor. Appl. Genet. 125, 1797–1808 (2012).
Beckman, P. M. & Payne, G. A. External growth, penetration, and development of Cercospora zeae-maydis in corn leaves. Phytopathology 72, 810–815 (1982).
Liu, L. et al. QTL mapping for gray leaf spot resistance in a tropical maize population. Plant Dis. 100, 304–312 (2016).
Delteil, A. et al. Several wall-associated kinases participate positively and negatively in basal defense against rice blast fungus. BMC Plant Biol. 16, 17 (2016).
Harkenrider, M. et al. Overexpression of rice wall-associated kinase 25 (OsWAK25) alters resistance to bacterial and fungal pathogens. PLoS ONE 11, e0147310 (2016).
Li, H., Zhou, S. Y., Zhao, W. S., Su, S. C. & Peng, Y. L. A novel wall-associated receptor-like protein kinase gene, OsWAK1, plays important roles in rice blast disease resistance. Plant Mol. Biol. 69, 337–346 (2009).
Rosli, H. G. et al. Transcriptomics-based screen for genes induced by flagellin and repressed by pathogen effectors identifies a cell wall-associated kinase involved in plant immunity. Genome Biol. 14, R139 (2013).
Brutus, A., Sicilia, F., Macone, A., Cervone, F. & De Lorenzo, G. A domain swap approach reveals a role of the plant wall-associated kinase 1 (WAK1) as a receptor of oligogalacturonides. Proc. Natl Acad. Sci. USA 107, 9452–9457 (2010).
Kohorn, B. D. et al. A dominant allele of Arabidopsis pectin-binding wall-associated kinase induces a stress response suppressed by MPK6 but not MPK3 mutations. Mol. Plant 5, 841–851 (2012).
Häffner, E., Karlovsky, P., Splivallo, R., Traczewska, A. & Diederichsen, E. ERECTA, salicylic acid, abscisic acid, and jasmonic acid modulate quantitative disease resistance of Arabidopsis thaliana to Verticillium longisporum. BMC Plant Biol. 14, 85 (2014).
Meier, S. et al. The Arabidopsis wall associated kinase-like 10 gene encodes a functional guanylyl cyclase and is co-expressed with pathogen defense related genes. PLoS ONE 5, e8904 (2010).
Yang, K., Qi, L. & Zhang, Z. Isolation and characterization of a novel wall-associated kinase gene TaWAK5 in wheat (Triticum aestivum). Crop J. 2, 255–266 (2014).
Dmochowska-Boguta, M. et al. TaWAK6 encoding wall-associated kinase is involved in wheat resistance to leaf rust similar to adult plant resistance. PLoS ONE 15, e0227713 (2020).
Wang, J. & Chai, J. Structural insights into the plant immune receptors PRRs and NLRs. Plant Physiol. 182, 1566–1581 (2020).
Sun, Y. et al. Structural basis for flg22-induced activation of the Arabidopsis FLS2-BAK1 immune complex. Science 342, 624–628 (2013).
Bender, K. W. et al. Activation loop phosphorylation of a non-RD receptor kinase initiates plant innate immune signaling. Proc. Natl Acad. Sci. USA 118, e2108242118 (2021).
Tang, D., Wang, G. & Zhou, J. M. Receptor kinases in plant-pathogen interactions: more than pattern recognition. Plant Cell 29, 618–637 (2017).
Ao, Y. et al. OsCERK1 and OsRLCK176 play important roles in peptidoglycan and chitin signaling in rice innate immunity. Plant J. 80, 1072–1084 (2014).
Wang, J. et al. The kinase OsCPK4 regulates a buffering mechanism that fine-tunes innate immunity. Plant Physiol. 176, 1835–1849 (2018).
Li, Z. et al. OsRLCK 57, OsRLCK107 and OsRLCK118 positively regulate chitin- and PGN-induced immunity in rice. Rice 10, 6 (2017).
Abuqamar, S., Chai, M. F., Luo, H., Song, F. & Mengiste, T. Tomato protein kinase 1b mediates signaling of plant responses to necrotrophic fungi and insect herbivory. Plant Cell 20, 1964–1983 (2008).
Li, L. et al. The FLS2-associated kinase BIK1 directly phosphorylates the NADPH oxidase RbohD to control plant immunity. Cell Host Microbe 15, 329–338 (2014).
Zhang, J. et al. Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe 7, 290–301 (2010).
Yamada, K., Yamaguchi, K., Yoshimura, S., Terauchi, A. & Kawasaki, T. Conservation of chitin-induced MAPK signaling pathways in rice and Arabidopsis. Plant Cell Physiol. 58, 993–1002 (2017).
Yamada, K. et al. The Arabidopsis CERK1-associated kinase PBL27 connects chitin perception to MAPK activation. EMBO J. 35, 2468–2483 (2016).
Kong, Q. et al. Two redundant receptor-like cytoplasmic kinases function downstream of pattern recognition receptors to regulate activation of SA biosynthesis. Plant Physiol. 171, 1344–1354 (2016).
Yan, H. et al. Brassinosteroid-signaling kinase 1 phosphorylates MAPKKK5 to regulate immunity in Arabidopsis. Plant Physiol. 176, 2991–3002 (2018).
Li, W., Liao, C.-J., Bluhm, B. H., Mengiste, T. & Woloshuk, C. P. A maize (Zea mays L.) BIK1-like receptor-like cytoplasmic kinase contributes to disease resistance. Plant Mol. Biol. Rep. 40, 28–42 (2021).
Kadota, Y. et al. Direct regulation of the NADPH oxidase RBOHD by the PRR-associated kinase BIK1 during plant immunity. Mol. Cell 54, 43–55 (2014).
Torres, M. A. & Dangl, J. L. Functions of the respiratory burst oxidase in biotic interactions, abiotic stress and development. Curr. Opin. Plant Biol. 8, 397–403 (2005).
Chen, Q. & Yang, G. Signal function studies of ROS, especially RBOH-dependent ROS, in plant growth, development and environmental stress. J. Plant Growth Regul. 39, 157–171 (2020).
Chen, W. et al. Convergent selection of a WD40 protein that enhances grain yield in maize and rice. Science 375, eabg7985 (2022).
Xing, H. L. et al. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 14, 327 (2014).
Liu, Q. et al. A helitron-induced RabGDIα variant causes quantitative recessive resistance to maize rough dwarf disease. Nat. Commun. 11, 495 (2020).
Ding, Y. et al. EGR2 phosphatase regulates OST1 kinase activity and freezing tolerance in Arabidopsis. EMBO J. 38, e99819 (2019).
Acknowledgements
We thank the members of Xu Laboratory for their helpful discussions. We thank the Center for Crop Functional Genomics and Molecular Breeding of China Agricultural University for providing transgenic technology support. We also thank W. Zhu (China Agricultural University) and G. Bi (China Agricultural University) for their insightful discussions during manuscript preparation. This research was supported by the National Key Research and Development Program (grant 2022YFD1201800) and the National Natural Science Foundation of China (grant 31471500).
Author information
Authors and Affiliations
Contributions
M.L.X. and T.Z. conceived the project and designed the experiments. M.L.X. supervised this project. T.Z. performed most of the experiments. M.Z. was involved in most of the fieldwork. Y.Z. and L.X. were involved in the fine-mapping process and helped revise the manuscript. Q.Q.Z. was involved in the phosphorylation assay and S.N.D. was involved in confocal microscopy experiments and protein expression. C.Y.G. identified the interaction between ZmBLK1 and ZmRBOH4. T.T.L. and Y.C.L. contributed to the materials management. X.M.F. and Y.Q.B. provided the materials and supported the phenotypic identification. P.B.-K. participated in project discussions and manuscript revision. M.L.X. and T.Z. wrote the manuscript with input from all authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks Matthew Moscou and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Sequential fine-mapping of qRgls1.
(a) Previous mapping efforts located qRgls1 to a 1.4-Mb region on the short arm of maize chromosome 8. (b–d) Sequential fine-mapping of qRgls1 (n = 3290). The schematic diagrams to the right represent the genomic architecture of each recombinant type. The markers used in the fine-mapping are indicated, with red markers showing the limits of the qRgls1 region as defined at each fine-mapping step. The tables to the right summarize the DSI from homozygous and heterozygous backcross progeny of recombinants. Significant difference (P < 0.05) in DSI between heterozygous and homozygous offspring indicates the presence of the resistance allele at qRgls1 in the heterozygous region of their parental recombinant; otherwise, there is no qRgls1 (P > 0.05). Data are shown as means ± s.d. in (b)–(d) and the P value was determined based on two-sided Student’s t-test.
Extended Data Fig. 2 The genetic effect and phenotype of qRgls1.
(a) Genetic effect of qRgls1 on maize resistance to gray leaf spot in field tests. (b,c) GLS symptoms (b) and GLS scale (c) (n = 98) of NIL-Q11 and NIL-Y32 plants at 44 dpi in the field. Scale bar, 15 cm. (d) C. zeina hyphae on the leaf surface of NIL-Q11 and NIL-Y32 plants at 44 dpi. Scale bars, 500 μm (overall view); 100 μm (detailed view). At least three separate leaves were observed with similar results. (e,f) Pairwise comparison of flowering-related traits of two NILs in Beijing (long days) (n = 5) and Sanya (short days) (n = 3). Data are shown as means ± s.d. in (a), means ± s.e. in (f). In (c), data are shown as violin plots with individual data points. In (a), (c) and (e) the P value was determined based on two-sided Student’s t-test. Data in (e) are displayed as box and whisker plots with individual data points. The error bars represent maximum and minimum values. Center line, median; box limits, 25th and 75th percentiles.
Extended Data Fig. 3 The schematic diagrams of ZmWAKL and ZmPR5L loci.
Schematic diagrams of ZmWAKL (a) and ZmPR5L (b) loci, as determined by aligning the full-size complementary DNA (cDNA) with the corresponding genomic sequence. Rectangles, exons; lines, introns. The colors indicate different domains: yellow, SP (signal peptide); light orange, GUB (galacturonan-binding domain of wall-associated receptor kinase); pink, TM (transmembrane domain); blue, Ser/Thr kinase. Gray shading between the Y32 and Q11 alleles represents the syntenic region; red lines denote SNPs; black spaces or lines represent InDels.
Extended Data Fig. 4 Transgenic functional validation of the susceptible ZmWAKLQ allele and the chimeric ZmWAKLC gene in GLS resistance.
(a) Schematic diagram of the Ubipro:ZmWAKLQ-GFP construct. (b) Relative expression of ZmWAKL in ZmWAKLQ overexpressing plants (n = 3). (c,d) GLS resistance of the ZmWAKLQ overexpression transgenic plants in backcross populations (n = 397, T1BC1F1; n = 455, T1BC3F1). (e) Schematic diagram of the Ubipro:ZmWAKLC construct. The chimeric ZmWAKLC gene was constructed by combining the ZmWAKLY ECD/TM domains (1-324 aa) in-frame with the ZmWAKLQ intracellular domain (364-704 aa). (f) Relative expression of ZmWAKL in ZmWAKLC overexpressing plants (n = 3). (g) GLS resistance of the ZmWAKLC overexpression transgenic plants in backcross populations (n = 918). (h) DSI values resulting from the overexpression of ZmWAKLY (n = 28) or ZmWAKLC (n = 38). Data are shown as means ± s.e. in (c), (d), (g) and (h); means ± s.d. in (b) and (f). Statistical significance was determined by a two-sided Student’s t-test in (b) and (f) or a paired t-test in (c), (d), (g) and (h).
Extended Data Fig. 5 Transgenic functional validation of ZmPR5L in GLS resistance.
(a) Relative expression of ZmPR5L in plants overexpressing ZmPR5L (n = 3). (b,c) GLS symptoms (b) and DSI (c) in segregating T2 transgenic plants (n = 365). N, non-transgenic plant; T, transgenic plant. Scale bars, 15 cm. (d) Schematic diagram of ZmPR5L and CRISPR/Cas9 editing target (red box). (e,f) GLS symptoms (e) and GLS scale (n = 148) (f) of the wild-type B73 and homozygous Zmpr5l knockout plants. Scale bar, 15 cm. Data are shown as means ± s.d. in (a); means in (c). Statistical significance was determined by a two-sided Student’s t-test in (a) and (c). Data in (f) are displayed as box and whisker plots, and different lowercase letters indicate a significant difference (P < 0.05) based on one-way ANOVA with Fisher’s least significant difference (LSD) test. The asterisk denotes the mean, and the box limits indicate the interquartile range.
Extended Data Fig. 6 Molecular characterization of ZmWAKL.
(a) Relative ZmWAKL expression in tissues collected from NIL-Q11 and NIL-Y32 plants (n = 3). (b,c) Relative expression levels of ZmWAKL in NIL-Q11 and NIL-Y32 after inoculation with C. zeina in the greenhouse (b) or field (c). (d,e) Subcellular localization of ZmWAKL in onion epidermal cells (d) and ZmWAKL-GFP overexpression transgenic plants (e). Plasmolysis was achieved by incubating cells in 0.3 g/mL sucrose for 5 min. Scale bars, 50 μm. DIC, differential interference contrast. The membrane was stained with the membrane-impermeable dye FM4-64. This experiment was repeated two times with the same results. In (a–c), values are means ± s.d., statistical significance was determined by a two-sided Student’s t-test.
Extended Data Fig. 7 ZmWIK interacts with ZmWAKLICD and regulates GLS resistance.
(a,c) Interactions of plasma membrane-tethered kinases with two ZmWAKL isoforms in SLC assays. (b,d) Analysis of relative luminescence units (RLUs) representing the interaction strengths between two ZmWAKL isoforms and plasma membrane-tethered kinases in pairwise combinations (n = 8). (e) Relative expression of ZmPK2 and ZmWIK in leaves of NILs (n = 3). (f,g) Plasma-membrane localization of ZmWIK in onion epidermal cells (f) and N. benthamiana leaves (g). Plasmolysis was achieved by incubating onion epidermal cells in 0.3 g/mL sucrose for 5 min. In N. benthamiana leaves, AtPIP2-mCherry (Plasma membrane intrinsic protein 2) was used as the membrane localization marker. DIC, differential interference contrast. Scale bars, 50 μm (onion epidermal cells); 100 μm (N. benthamiana leaves). This experiment was repeated two times with the same results. (h) Relative expression of ZmWIK in B73 and two homozygous overexpression transgenic lines (n = 3). (i,j) GLS symptoms (i) and scales (n = 265) (j) of B73 and transgenic lines. Scale bar, 15 cm. Data are shown as means ± s.d. in (b), (d), (e) and (h), statistical significance was determined by a two-sided Student’s t-test. Data in (j) are displayed as box and whisker plots with individual data points, the asterisk denotes the mean and the box limits indicate the interquartile range. In (b), (d) and (h), different lowercase letters indicate a significant difference (P < 0.05) based on one-way ANOVA with Tukey’s test. In (j), different lowercase letters indicate a significant difference (P < 0.05) based on one-way ANOVA with LSD test. The SLC assays were repeated at least four times.
Extended Data Fig. 8 ZmBLK1 interacts with the ZmWAKL/ZmWIK complex for phosphorylation signaling.
(a) Determination of the interaction between ZmBLK1/ZmBLK1-1 and ZmWAKL or ZmWIK. (b) Phosphorylation of ZmBLK1 by ZmWAKLY/ZmWIK or ZmWAKLQ/ZmWIK in N. benthamiana leaves. ZmBLK1 was immunoprecipitated with α-Myc magnetic beads (IP, α-Myc). The immunoprecipitated and input samples were immunoblotted with the indicated antibodies. α-pS/T, anti-phospho-(Ser/Thr) antibody. (c) Phosphorylation of ZmBLK1 by the mixed ZmWAKLY/ZmWIK and ZmWAKLQ/ZmWIK in vivo. ZmBLK1 was immunoprecipitated with α-Myc magnetic beads (IP, α-Myc). The immunoprecipitated and input samples were immunoblotted with the indicated antibodies. α-pS/T, anti-Phospho-(Ser/Thr) antibody. (d) Determination of ZmBLK1 kinase activity. The immunoprecipitated ZmBLK1 in (c) can phosphorylate the substrate MBPa. Green ‘Y’ and blue ‘Q’ denote ZmBLK1 immunoprecipitated from the protoplasts of Ubipro:ZmWAKLY-GFP and Ubipro:ZmWAKLQ-GFP, respectively. Phosphorylation of MBPa was detected by autoradiography (upper). Equal protein loading is shown by CBB staining (lower). The protein amounts were quantified by densitometry of band intensities, with the value to the right set to 1. The SLC assays were repeated at least two times and the in vivo kinase assays were repeated two times.
Extended Data Fig. 9 Investigation of the interactions between N-ZmRBOH4 and ZmWAKL (or ZmWIK).
(a) Phylogenetic analysis demonstrated that ZmRBOH4 is closely related to AtRBOHD. (b) Determination of the interaction between ZmBLK1/ZmBLK1-1 and ZmRBOH4. (c,d) N-ZmRBOH4 (1-377aa) does not interact with ZmWAKLY, ZmWAKLQ or ZmWIK in a SLC assay (c) or GST pull-down assay in vitro (d). (e) Interactions of cLUC-ZmBLK1 with ZmRBOHs-nLUC in an SLC assay. (f) Relative expression levels of ZmRBOHs in NIL-Q11 and NIL-Y32 after inoculation with C. zeina (n = 3). hpi, hours post inoculation. The SLC and pull-down assays were repeated at least three times.
Extended Data Fig. 10 Knockout of ZmRBOH4 by CRISPR/Cas9 results in changes to plant morphology.
(a) Schematic diagram of ZmRBOH4 and the sgRNA target site in the first exon for CRISPR/Cas9-mediated editing. The sequences of two homozygous knockout lines with insertion (ko #1) and deletion (ko #2) are shown. The wild-type B73 sequence is shown on top. The target sites and PAM are highlighted in red and gray, respectively, with deletions indicated by dashes. (b,c) Plant morphology (b) and height (c) of B73 and two homozygous knockout lines (n = 183). The knockout lines exhibit significantly reduced height. Scale bar, 30 cm. Data in (c) are displayed as box and whisker plots with individual data points, the asterisk denotes the mean, and the box limits indicate the interquartile range. The center line indicates the median, and different lowercase letters indicate a significant difference (P < 0.05) based on one-way ANOVA with LSD test.
Supplementary information
Supplementary Information
Supplementary Figs. 1–8, Supplementary Methods, Supplementary Tables 1–5 and Supporting data for Supplementary Figs. 1 and 6.
Supplementary Data
Statistical supporting data of Supplementary Figs. 1, 3, 5, 6 and 8.
Source data
Source Data Fig. 1
Statistical source data.
Source Data Fig. 3
Statistical source data.
Source Data Fig. 4
Statistical source data.
Source Data Fig. 5
Statistical source data.
Source Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 1
Statistical source data.
Source Data Extended Data Fig. 2
Statistical source data.
Source Data Extended Data Fig. 4
Statistical source data.
Source Data Extended Data Fig. 5
Statistical source data.
Source Data Extended Data Fig. 6
Statistical source data.
Source Data Extended Data Fig. 7
Statistical source data.
Source Data Extended Data Fig. 9
Statistical source data.
Source Data Extended Data Fig. 10
Statistical source data.
Source Data Figs. 1–6 and Extended Data Figs. 4, 8, and 9
Unprocessed western blots and gels.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhong, T., Zhu, M., Zhang, Q. et al. The ZmWAKL–ZmWIK–ZmBLK1–ZmRBOH4 module provides quantitative resistance to gray leaf spot in maize. Nat Genet 56, 315–326 (2024). https://doi.org/10.1038/s41588-023-01644-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-023-01644-z
This article is cited by
-
The symphony of maize signaling quartet defending against gray leaf spot
Stress Biology (2024)
-
The maize ZmCPK39-ZmKnox2 module regulates plant height
aBIOTECH (2024)