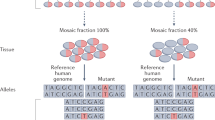
Abstract
Somatic mosaicism is a known cause of neurological disorders, including developmental brain malformations and epilepsy. Brain mosaicism is traditionally attributed to post-zygotic genetic alterations arising in fetal development. Here we describe post-zygotic rescue of meiotic errors as an alternate origin of brain mosaicism in patients with focal epilepsy who have mosaic chromosome 1q copy number gains. Genomic analysis showed evidence of an extra parentally derived chromosome 1q allele in the resected brain tissue from five of six patients. This copy number gain is observed only in patient brain tissue, but not in blood or buccal cells, and is strongly enriched in astrocytes. Astrocytes carrying chromosome 1q gains exhibit distinct gene expression signatures and hyaline inclusions, supporting a novel genetic association for astrocytic inclusions in epilepsy. Further, these data demonstrate an alternate mechanism of brain chromosomal mosaicism, with parentally derived copy number gain isolated to brain, reflecting rescue in other tissues during development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The single-nuclei RNA-seq data presented in this publication has been deposited in NCBI Gene Expression Omnibus and is accessible through GEO Series accession number GSE221849. Exome sequencing data are available either through dbGAP (phs002128.v1.p1) or BioProject (PRJNA1017875). Capture and targeted sequencing results are available as supplementary data. Exome sequence data from 342 controls were obtained from the North American Brain Expression Consortium (NABEC—dbGAP Study Accession: phs001300.v1.p1), and 15 whole genome-sequenced controls available from the Brain Somatic Mosaicism Network (National Institute of Mental Health Data Archive). Human reference genome build GRCh38.p12 is available from Index of /genomes/all/GCF/000/001/405/GCF_000001405.26_GRCh38 (https://www.nih.gov/). The Allen Brain Map Human M1 Cortex Dataset is available from https://portal.brain-map.org/atlases-and-data/rnaseq/human-m1-10x.
Code availability
R scripts to analyze the data and generate the figures are available on GitHub (https://github.com/bedrosian-lab/1q_mosaicism).
References
Bae, T. et al. Different mutational rates and mechanisms in human cells at pregastrulation and neurogenesis. Science 359, 550–555 (2018).
D’Gama, A. M. & Walsh, C. A. Somatic mosaicism and neurodevelopmental disease. Nat. Neurosci. 21, 1504–1514 (2018).
Kobow, K. et al. Mosaic trisomy of chromosome 1q in human brain tissue associates with unilateral polymicrogyria, very early-onset focal epilepsy, and severe developmental delay. Acta Neuropathol. 140, 881–891 (2020).
Poduri, A. et al. Somatic activation of AKT3 causes hemispheric developmental brain malformations. Neuron 74, 41–48 (2012).
Bedrosian, T. A. et al. Detection of brain somatic variation in epilepsy-associated developmental lesions. Epilepsia 63, 1981–1997 (2022).
Lai, D. et al. Somatic variants in diverse genes leads to a spectrum of focal cortical malformations. Brain 145, 2704–2720 (2022).
Fischer, G. M. et al. Filamin A-negative hyaline astrocytic inclusions in pediatric patients with intractable epilepsy: report of 2 cases. J. Neurosurg. Pediatr. 26, 38–44 (2020).
Hedley-Whyte, E. T. et al. Hyaline protoplasmic astrocytopathy of neocortex. J. Neuropathol. Exp. Neurol. 68, 136–147 (2009).
Cai, X. et al. Single-cell, genome-wide sequencing identifies clonal somatic copy-number variation in the human brain. Cell Rep. 10, 645 (2015).
Lopez-Rivera, J. A. et al. The genomic landscape across 474 surgically accessible epileptogenic human brain lesions. Brain 146, 1342–1356 (2023).
Conlin, L. K. et al. Mechanisms of mosaicism, chimerism and uniparental disomy identified by single nucleotide polymorphism array analysis. Hum. Mol. Genet. 19, 1263–1275 (2010).
Robberecht, C. et al. Meiotic errors followed by two parallel postzygotic trisomy rescue events are a frequent cause of constitutional segmental mosaicism. Mol. Cytogenet. 5, 19 (2012).
Patel, A. P. et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344, 1396–1401 (2014).
Pai, B. et al. High-resolution transcriptomics informs glial pathology in human temporal lobe epilepsy. Acta Neuropathol. Commun. 10, 149 (2022).
Satz, J. S. et al. Distinct functions of glial and neuronal dystroglycan in the developing and adult mouse brain. J. Neurosci. 30, 14560–14572 (2010).
Verhoog, Q. P., Holtman, L., Aronica, E. & van Vliet, E. A. Astrocytes as guardians of neuronal excitability: mechanisms underlying epileptogenesis. Front. Neurol. 11, 591690 (2020).
Saint-Martin, M. & Goda, Y. Astrocyte-synapse interactions and cell adhesion molecules. FEBS J. 290, 3512–3526 (2023).
Miller, M. B. et al. Somatic genomic changes in single Alzheimer’s disease neurons. Nature 604, 714–722 (2022).
Chen, C. P. et al. Mosaic tetrasomy 12p with discrepancy between fetal tissues and extraembryonic tissues: molecular analysis and possible mechanism of formation. Taiwan J. Obstet. Gynecol. 49, 235–238 (2010).
Coorens, T. H. H. et al. Inherent mosaicism and extensive mutation of human placentas. Nature 592, 80–85 (2021).
Eggermann, T. et al. Mosaic trisomy 1q due to a de novo translocation in a foetus with early developmental abnormalities (karyotype 46,XY, der(14),t(1;14)(p11;p11.2)/46,XY) delineation of parent and cell stage of origin. Int. J. Hum. Genet. 8, 317–323 (2008).
Scheres, J. M. et al. Isochromosome 1q as the sole chromosomal abnormality in two fetal teratomas. Possible trisomic or tetrasomic zygote rescue in fetal teratoma with an additional isochromosome 1q. Cancer Genet. Cytogenet. 115, 1–10 (1999).
Warburton, D. et al. Trisomy recurrence: a reconsideration based on North American data. Am. J. Hum. Genet. 75, 376–385 (2004).
Struski, S., Doco-Fenzy, M. & Cornillet-Lefebvre, P. Compilation of published comparative genomic hybridization studies. Cancer Genet. Cytogenet. 135, 63–90 (2002).
Vladoiu, M. C. et al. Childhood cerebellar tumours mirror conserved fetal transcriptional programs. Nature 572, 67–73 (2019).
Ward, S. et al. Gain of 1q and loss of 22 are the most common changes detected by comparative genomic hybridisation in paediatric ependymoma. Genes Chromosomes Cancer 32, 59–66 (2001).
Carter, M. et al. Genetic abnormalities detected in ependymomas by comparative genomic hybridisation. Br. J. Cancer 86, 929–939 (2002).
Pajtler, K. W. et al. Molecular classification of ependymal tumors across all CNS compartments, histopathological grades, and age groups. Cancer Cell 27, 728–743 (2015).
Varela, C. et al. Recurrent genomic instability of chromosome 1q in neural derivatives of human embryonic stem cells. J. Clin. Invest. 122, 569–574 (2012).
Mehrjardi, N. Z. et al. Acquisition of chromosome 1q duplication in parental and genome-edited human-induced pluripotent stem cell-derived neural stem cells results in their higher proliferation rate in vitro and in vivo. Cell Prolif. 53, e12892 (2020).
Minagawa, M., Shioda, K., Shimizu, Y. & Isshiki, T. Inclusion bodies in cerebral cortical astrocytes: a new change of astrocytes. Acta Neuropathol. 84, 113–116 (1992).
Van den Veyver, I. B. et al. Presence of filamin in the astrocytic inclusions of Aicardi syndrome. Pediatr. Neurol. 30, 7–15 (2004).
Barba, C. et al. Clinical features, neuropathology, and surgical outcome in patients with refractory epilepsy and brain somatic variants in the SLC35A2 gene. Neurology 100, e528–e542 (2023).
Kelly, B. J. et al. Churchill: an ultra-fast, deterministic, highly scalable and balanced parallelization strategy for the discovery of human genetic variation in clinical and population-scale genomics. Genome Biol. 16, 6 (2015).
Koboldt, D. C. et al. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 22, 568–576 (2012).
Koboldt, D. C. et al. PTEN somatic mutations contribute to spectrum of cerebral overgrowth. Brain 144, 2971–2978 (2021).
McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010).
Miller, K. E. et al. Somatic SLC35A2 mosaicism correlates with clinical findings in epilepsy brain tissue. Neurol. Genet. 6, e460 (2020).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Hao, Y. et al. Integrated analysis of multimodal single-cell data. Cell 184, 3573–87.e29 (2021).
McGinnis, C. S., Murrow, L. M. & Gartner, Z. J. DoubletFinder: doublet detection in single-cell RNA sequencing data using artificial nearest neighbors. Cell Syst. 8, 329–37.e4 (2019).
InferCNV. GitHub https://github.com/broadinstitute/inferCNV (2020).
Finak, G. et al. MAST: a flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. Genome Biol. 16, 278 (2015).
Wu, T. et al. clusterProfiler 4.0: a universal enrichment tool for interpreting omics data. Innovation 2, 100141 (2021).
Acknowledgements
We thank the patients and families for contributing their specimens to advance our understanding of the genetic bases of epilepsy-associated brain malformations. We also thank R. K. Wilson and the Genomic Services Laboratory and Computational Genomics Group at Nationwide Children’s Hospital. The work was funded by the Nationwide Innovation Fund, L. B. Research and Education Foundation (N.S.), The Ohio State University Medical Scientist Training Program—T32 Training Grant—T32GM139784 (N.S.), NIH-NINDS (R01NS094596) to E.L.H., and NIH-NINDS (R01NS129784) to T.A.B. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Some figures were created in BioRender. Data and/or research tools used in the preparation of this manuscript were obtained from the National Institute of Mental Health Data Archive. National Institute of Mental Health Data Archive is a collaborative informatics system created by the National Institutes of Health to provide a national resource to support and accelerate research in mental health. Dataset identifier(s): 2962. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or of the Submitters submitting original data to the National Institute of Mental Health Data Archive.
Author information
Authors and Affiliations
Contributions
K.E.M., E.L.H. and T.A.B. conceptualized the study. K.E.M., E.L.H., T.A.B., J.B.N., J.J.W., A.C.R., N.S., R.D.R., M.G., M.M., S.S., D.C.K. and A.R.M. performed the experiments and analyzed data. K.E.M., T.A.B., N.S. and A.C.R. visualized the results. T.A.B., E.L.H. and E.R.M. contributed funding and resources. K.E.M., E.L.H., T.A.B., D.C.K. and R.S. contributed project administration support. Y.A., D.R.B., D.L.T., C.R.P., J.P., A.S., J. Lu, J. Leonard, A.P.O., A.P., C.H., M.E.H., E.L.H., M.B.B., O.R., E.Y., H.G.W.L., M.N.S., G.K.V.A., J.E.G. and D.C.K. provided patient data and samples, provided phenotypic data and interpreted clinical findings. K.E.M., D.C.K., E.L.H. and T.A.B. supervised the research. K.E.M., E.L.H. and T.A.B. wrote the original draft. All authors participated in review and editing of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Genetics thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Single-nuclei whole genome sequencing.
Whole genome sequencing of single-nuclei from Patient 1 brain tissue confirming the presence of chromosome 1q tetrasomy. Chromosome 1 copy number data is shown for representative nuclei with and without 1q tetrasomy (top) and the entire genome is shown (bottom) for the cell with the 1q gain.
Extended Data Fig. 2 Parental origin of chromosome 1q SNVs.
a, Variant allele fractions for SNVs found on chromosome 1q in Patient 1 brain, blood, buccal, and parental blood samples, demonstrating that these SNVs represent an extra chromosome 1q allele of maternal origin. b, OncoScan array results for Patient 1 trio demonstrating a chromosome 1q gain in proband brain tissue but no gain observed in blood samples from either parent, showing the parents are karyotypically normal. c, Variant allele fractions for a small number of representative chromosome 1q SNVs in available tissues from Patients 4, 5, and 6.
Extended Data Fig. 3 Mosaic chromosome 1q SNVs in patients with epilepsy or tumors.
a, Number of brain-restricted mosaic variants identified from exome sequencing of resected brain tissue versus blood samples from epilepsy Patients 1–6. b, Somatic variants identified from tumor-normal exome sequencing of various pediatric CNS tumors with chromosome 1q gains. No enrichment of variants on chromosome 1q is observed.
Extended Data Fig. 4 Inverted duplication of chromosome 1q in one patient.
a, A breakpoint-spanning read from PacBio HiFi long-read sequencing of Patient 2 brain DNA identifies the structure of the gain as an inverted duplication of chromosome 1q. b, Optical genome mapping coverage of the breakpoint.
Extended Data Fig. 5 Single-nuclei RNA-seq.
a, Single-nuclei RNA-seq quality control metrics: number of reads per nucleus, number of genes per nucleus, and percent mitochondrial reads. b, Feature plots showing expression of cell type markers.
Extended Data Fig. 6 Chromosome 1q gain in astrocytes.
a, InferCNV output for Patient 3 demonstrating the chromosome 1q gain enriched in astrocytes. b, Percent of cells with chromosome 1q gain depicted by patient and by cell type. c, Astrocytes with and without the chromosome 1q gain highly express astrocytic marker genes. d, InferCNV output comparing Patient 3 astrocytes from affected brain tissue to Patient 3 astrocytes from unaffected adjacent brain tissue. e, Fluorescence activated nuclei sorting based on PAX6-APC signal to enrich astrocytes followed by targeted sequencing of chromosome 1q marker SNVs in Patient 3. Astrocyte enriched cell population has increased representation of the chromosome 1q gain compared to bulk cells or PAX6-negative cells.
Extended Data Fig. 7 Gene expression differences between excitatory neurons with and without chromosome 1q gain.
a, Volcano plot showing differentially expressed genes in excitatory neurons with the chromosome 1q gain vs. those without. Black points represent genes located on chromosome 1q. b, Enriched GO terms for 1q gain excitatory neurons derived from an over-representation test performed with the enrichGO function of clusterprofiler R package with FDR p-value adjustment. c, Venn diagram showing overlap of differentially expressed genes with genes located on chromosome 1q.
Extended Data Fig. 8 Alternate models of isochromosome formation.
a, A potential model of mosaic isochromosome 1q formation in the early cleavage-stage embryo. b, An alternate model of mosaic isochromosome 1q formation originating in an oocyte.
Extended Data Fig. 9 Neuroimaging.
Brain MRIs from subjects with 1q duplications. a, b, Axial (a) and coronal (b) T2 images of Patient 1 at 13 months demonstrates hazy T2 prolongation involving the right frontal and parietal lobes (dashed circles). c, d, Axial (c) and coronal (d) T2 images of Patient 2 at 5 years of age demonstrate a dysplastic right frontal sulcus (dashed circles). e, f, Axial (e) and coronal (f) T2 images of Patient 3 at 9 months of age demonstrate polymicrogyria in the right frontal lobe and insula (dashed circle). g, h, Axial T1 (g) and axial T2 (h) images of Patient 6 at 14 months demonstrates hazy T1/T2 prolongation involving the inferior left frontal lobe (dashed circles). i, j, Axial T2 (i) and sagittal T1 (j) images of Patient 4 at 9 years of age demonstrate generalized decrease in size of the right hemisphere with a large frontoparietal area of dysplastic cortex (hazy T2 prolongation and increased gyral frequency indicated by dashed circles). k, l, Axial T2 (k) and coronal T2 (l) images of Patient 5 at 5 years of age demonstrate subtle T2 prolongation in the left supramarginal and superior temporal gyri (dashed circles).
Supplementary information
Supplementary Information
Supplementary Fig. 1.
Supplementary Data 1
1q mosaic variants identified in exome sequencing.
Supplementary Data 2
1q capture panel results for patient 3.
Supplementary Data 3
1q capture panel results for patient 1.
Supplementary Data 4
Deep targeted amplicon sequencing results for patients 1, 3, 4, 5 and 6.
Supplementary Data 5
Number of 1q gain versus no gain cells used for differential expression analysis per cell type and lists of differentially expressed genes identified per cell type.
Supplementary Data 6
Oligonucleotide sequences used for targeted sequencing assays.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Miller, K.E., Rivaldi, A.C., Shinagawa, N. et al. Post-zygotic rescue of meiotic errors causes brain mosaicism and focal epilepsy. Nat Genet 55, 1920–1928 (2023). https://doi.org/10.1038/s41588-023-01547-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-023-01547-z
This article is cited by
-
Technological Vanguard: the outstanding performance of the LTY-CNN model for the early prediction of epileptic seizures
Journal of Translational Medicine (2024)
-
Human embryonic genetic mosaicism and its effects on development and disease
Nature Reviews Genetics (2024)
-
Post-zygotic brain mosaicism as a result of partial reversion of pre-zygotic aneuploidy
Nature Genetics (2023)