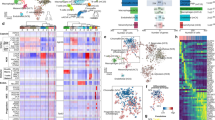
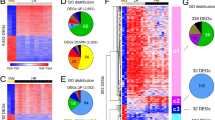
Abstract
Neuroblastoma is a pediatric tumor of the developing sympathetic nervous system. However, the cellular origin of neuroblastoma has yet to be defined. Here we studied the single-cell transcriptomes of neuroblastomas and normal human developing adrenal glands at various stages of embryonic and fetal development. We defined normal differentiation trajectories from Schwann cell precursors over intermediate states to neuroblasts or chromaffin cells and showed that neuroblastomas transcriptionally resemble normal fetal adrenal neuroblasts. Importantly, neuroblastomas with varying clinical phenotypes matched different temporal states along normal neuroblast differentiation trajectories, with the degree of differentiation corresponding to clinical prognosis. Our work highlights the roles of oncogenic MYCN and loss of TFAP2B in blocking differentiation and may provide the basis for designing therapeutic interventions to overcome differentiation blocks.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
Raw adrenal gland and neuroblastoma sequencing data have been deposited in the European Genome-phenome Archive under study accession no. EGAS00001004388. Processed data can be downloaded and explored interactively in a Shiny App: https://adrenal.kitz-heidelberg.de/developmental_programs_NB_viz/. Raw and processed sequencing data for the neuroblastoma cell lines have been deposited in the Gene Expression Omnibus (GEO) under accession no. GSE163431. Bulk RNA-seq data of neuroblastomas from the SEQC cohort were obtained from the GEO (accession no. GSE49711). Bulk RNA-seq data of neuroblastomas from the TARGET cohort were obtained from https://portal.gdc.cancer.gov/projects/TARGET-NBL. The single-cell RNA-seq data of the neuroblastoma cell line SK-N-SH were obtained from the GEO under accession no. GSE158130. Expression data of IMR-32 cells on inducible overexpression of TFAP2B were obtained from the GEO under accession no. GSE74350. Whole-genome sequencing data for tumors studied by scRNA-seq were obtained from the European Genome-phenome Archive (accession nos. EGAS00001004349 and EGAS00001001308). Matching tumor IDs are provided in Supplementary Table 8. Source data are provided with this paper.
References
Maris, J. M., Hogarty, M. D., Bagatell, R. & Cohn, S. L. Neuroblastoma. Lancet 369, 2106–2120 (2007).
Peifer, M. et al. Telomerase activation by genomic rearrangements in high-risk neuroblastoma. Nature 526, 700–704 (2015).
Brodeur, G. M., Seeger, R. C., Schwab, M., Varmus, H. E. & Bishop, J. M. Amplification of N-myc in untreated human neuroblastomas correlates with advanced disease stage. Science 224, 1121–1124 (1984).
Ackermann, S. et al. A mechanistic classification of clinical phenotypes in neuroblastoma. Science 362, 1165–1170 (2018).
Mossé, Y. P. et al. Identification of ALK as a major familial neuroblastoma predisposition gene. Nature 455, 930–935 (2008).
Janoueix-Lerosey, I. et al. Somatic and germline activating mutations of the ALK kinase receptor in neuroblastoma. Nature 455, 967–970 (2008).
van Groningen, T. et al. Neuroblastoma is composed of two super-enhancer-associated differentiation states. Nat. Genet. 49, 1261–1266 (2017).
Boeva, V. et al. Heterogeneity of neuroblastoma cell identity defined by transcriptional circuitries. Nat. Genet. 49, 1408–1413 (2017).
Anderson, D. J., Carnahan, J. F., Michelsohn, A. & Patterson, P. H. Antibody markers identify a common progenitor to sympathetic neurons and chromaffin cells in vivo and reveal the timing of commitment to neuronal differentiation in the sympathoadrenal lineage. J. Neurosci. 11, 3507–3519 (1991).
De Preter, K. et al. Human fetal neuroblast and neuroblastoma transcriptome analysis confirms neuroblast origin and highlights neuroblastoma candidate genes. Genome Biol. 7, R84 (2006).
Furlan, A. et al. Multipotent peripheral glial cells generate neuroendocrine cells of the adrenal medulla. Science 357, eaal3753 (2017).
Cooper, M. J., Hutchins, G. M. & Israel, M. A. Histogenesis of the human adrenal medulla. An evaluation of the ontogeny of chromaffin and nonchromaffin lineages. Am. J. Pathol. 137, 605–615 (1990).
Molenaar, W. M., Lee, V. M. & Trojanowski, J. Q. Early fetal acquisition of the chromaffin and neuronal immunophenotype by human adrenal medullary cells. An immunohistological study using monoclonal antibodies to chromogranin A, synaptophysin, tyrosine hydroxylase, and neuronal cytoskeletal proteins. Exp. Neurol. 108, 1–9 (1990).
Magro, G. & Grasso, S. Immunohistochemical identification and comparison of glial cell lineage in foetal, neonatal, adult and neoplastic human adrenal medulla. Histochem. J. 29, 293–299 (1997).
Katsetos, C. D. et al. Class III β-tubulin isotype (β III) in the adrenal medulla: I. Localization in the developing human adrenal medulla. Anat. Rec. 250, 335–343 (1998).
Lake, B. B. et al. Integrative single-cell analysis of transcriptional and epigenetic states in the human adult brain. Nat. Biotechnol. 36, 70–80 (2018).
La Manno, G. et al. Molecular diversity of midbrain development in mouse, human, and stem cells. Cell 167, 566–580.e19 (2016).
Zhong, S. et al. Decoding the development of the human hippocampus. Nature 577, 531–536 (2020).
Gartlgruber, M. et al. Super enhancers define regulatory subtypes and cell identity in neuroblastoma. Nat. Cancer 2, 114–128 (2021).
Zimmerman, M. W. et al. MYC drives a subset of high-risk pediatric neuroblastomas and is activated through mechanisms including enhancer hijacking and focal enhancer amplification. Cancer Discov. 8, 320–335 (2018).
Ikram, F. et al. Transcription factor activating protein 2 beta (TFAP2B) mediates noradrenergic neuronal differentiation in neuroblastoma. Mol. Oncol. 10, 344–359 (2016).
Zhang, W. et al. Comparison of RNA-seq and microarray-based models for clinical endpoint prediction. Genome Biol. 16, 133 (2015).
Ambros, I. M. et al. Role of ploidy, chromosome 1p, and Schwann cells in the maturation of neuroblastoma. N. Engl. J. Med. 334, 1505–1511 (1996).
Wei, J. S. et al. Clinically relevant cytotoxic immune cell signatures and clonal expansion of T-cell receptors in high-risk MYCN-not-amplified human neuroblastoma. Clin. Cancer Res. 24, 5673–5684 (2018).
Sidell, N. Retinoic acid-induced growth inhibition and morphologic differentiation of human neuroblastoma cells in vitro. J. Natl Cancer Inst. 68, 589–596 (1982).
Matthay, K. K. et al. Long-term results for children with high-risk neuroblastoma treated on a randomized trial of myeloablative therapy followed by 13-cis-retinoic acid: a children’s oncology group study. J. Clin. Oncol. 27, 1007–1013 (2009).
Huber, K. The sympathoadrenal cell lineage: specification, diversification, and new perspectives. Dev. Biol. 298, 335–343 (2006).
Dong, R. et al. Single-cell characterization of malignant phenotypes and developmental trajectories of adrenal neuroblastoma. Cancer Cell 38, 716–733.e6 (2020).
Huber, K. Segregation of neuronal and neuroendocrine differentiation in the sympathoadrenal lineage. Cell Tissue Res. 359, 333–341 (2015).
Lumb, R. & Schwarz, Q. Sympathoadrenal neural crest cells: the known, unknown and forgotten? Dev. Growth Differ. 57, 146–157 (2015).
Janoueix-Lerosey, I., Lopez-Delisle, L., Delattre, O. & Rohrer, H. The ALK receptor in sympathetic neuron development and neuroblastoma. Cell Tissue Res. 372, 325–337 (2018).
Unsicker, K., Huber, K., Schober, A. & Kalcheim, C. Resolved and open issues in chromaffin cell development. Mech. Dev. 130, 324–329 (2013).
Ernst, K. J. Establishment of a simplified preparation method for single-nucleus RNA-sequencing and its application to long-term frozen tumor tissues. Preprint at bioRxiv https://doi.org/10.1101/2020.10.23.351809 (2020).
Jaitin, D. A. et al. Massively parallel single-cell RNA-seq for marker-free decomposition of tissues into cell types. Science 343, 776–779 (2014).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Tirosh, I. et al. Single-cell RNA-seq supports a developmental hierarchy in human oligodendroglioma. Nature 539, 309–313 (2016).
Angerer, P. et al. destiny: diffusion maps for large-scale single-cell data in R. Bioinformatics 32, 1241–1243 (2016).
Street, K. et al. Slingshot: cell lineage and pseudotime inference for single-cell transcriptomics. BMC Genomics 19, 477 (2018).
Trapnell, C. et al. The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol. 32, 381–386 (2014).
Qiu, X. et al. Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods 14, 979–982 (2017).
Cao, J. et al. The single-cell transcriptional landscape of mammalian organogenesis. Nature 566, 496–502 (2019).
La Manno, G. et al. RNA velocity of single cells. Nature 560, 494–498 (2018).
Bergen, V., Lange, M., Peidli, S., Wolf, F. A. & Theis, F. J. Generalizing RNA velocity to transient cell states through dynamical modeling. Nat. Biotechnol. 38, 1408–1414 (2020).
Teschendorff, A. E. & Enver, T. Single-cell entropy for accurate estimation of differentiation potency from a cell’s transcriptome. Nat. Commun. 8, 15599 (2017).
Gulati, G. S. et al. Single-cell transcriptional diversity is a hallmark of developmental potential. Science 367, 405–411 (2020).
Aibar, S. et al. SCENIC: single-cell regulatory network inference and clustering. Nat. Methods 14, 1083–1086 (2017).
Patel, A. P. et al. Single-cell RNA-seq highlights intratumoral heterogeneity in primary glioblastoma. Science 344, 1396–1401 (2014).
Aran, D. et al. Reference-based analysis of lung single-cell sequencing reveals a transitional profibrotic macrophage. Nat. Immunol. 20, 163–172 (2019).
Baron, M. et al. A single-cell transcriptomic map of the human and mouse pancreas reveals inter- and intra-cell population structure. Cell Syst. 3, 346–360.e4 (2016).
Newman, A. M. et al. Robust enumeration of cell subsets from tissue expression profiles. Nat. Methods 12, 453–457 (2015).
Muth, D. et al. Transcriptional repression of SKP2 is impaired in MYCN-amplified neuroblastoma. Cancer Res. 70, 3791–3802 (2010).
Henrich, K.-O. et al. Integrative genome-scale analysis identifies epigenetic mechanisms of transcriptional deregulation in unfavorable neuroblastomas. Cancer Res. 76, 5523–5537 (2016).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Tarasov, A., Vilella, A. J., Cuppen, E., Nijman, I. J. & Prins, P. Sambamba: fast processing of NGS alignment formats. Bioinformatics 31, 2032–2034 (2015).
DeLuca, D. S. et al. RNA-SeQC: RNA-seq metrics for quality control and process optimization. Bioinformatics 28, 1530–1532 (2012).
Liao, Y., Smyth, G. K. & Shi, W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 30, 923–930 (2014).
McCarthy, D. J., Chen, Y. & Smyth, G. K. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 40, 4288–4297 (2012).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
Robinson, M. D. & Oshlack, A. A scaling normalization method for differential expression analysis of RNA-seq data. Genome Biol. 11, R25 (2010).
Pihlajoki, M., Dörner, J., Cochran, R. S., Heikinheimo, M. & Wilson, D. B. Adrenocortical zonation, renewal, and remodeling. Front. Endocrinol. (Lausanne) 6, 27 (2015).
Goncharov, N. V., Nadeev, A. D., Jenkins, R. O. & Avdonin, P. V. Markers and biomarkers of endothelium: when something is rotten in the state. Oxid. Med. Cell. Longev. 2017, 9759735 (2017).
Xie, T. et al. Single-cell deconvolution of fibroblast heterogeneity in mouse pulmonary fibrosis. Cell Rep. 22, 3625–3640 (2018).
Foote, A. G., Wang, Z., Kendziorski, C. & Thibeault, S. L. Tissue specific human fibroblast differential expression based on RNAsequencing analysis. BMC Genomics 20, 308 (2019).
Donovan, J. A. & Koretzky, G. A. CD45 and the immune response. J. Am. Soc. Nephrol. 4, 976–985 (1993).
Irving, B. A., Chan, A. C. & Weiss, A. Functional characterization of a signal transducing motif present in the T cell antigen receptor zeta chain. J. Exp. Med. 177, 1093–1103 (1993).
Kadowaki, T. et al. Reconsideration of macrophage and dendritic cell classification. Anticancer Res. 32, 2257–2261 (2012).
Affer, M. et al. Gene expression differences between enriched normal and chronic myelogenous leukemia quiescent stem/progenitor cells and correlations with biological abnormalities. J. Oncol. 2011, 798592 (2011).
Castiglioni, I. et al. The Trithorax protein Ash1L promotes myoblast fusion by activating Cdon expression. Nat. Commun. 9, 5026 (2018).
Chal, J. & Pourquié, O. Making muscle: skeletal myogenesis in vivo and in vitro. Development 144, 2104–2122 (2017).
Robin, Y.-M. et al. Transgelin is a novel marker of smooth muscle differentiation that improves diagnostic accuracy of leiomyosarcomas: a comparative immunohistochemical reappraisal of myogenic markers in 900 soft tissue tumors. Mod. Pathol. 26, 502–510 (2013).
Hinz, B. et al. The myofibroblast: one function, multiple origins. Am. J. Pathol. 170, 1807–1816 (2007).
Hsia, L.-T. et al. Myofibroblasts are distinguished from activated skin fibroblasts by the expression of AOC3 and other associated markers. Proc. Natl Acad. Sci. USA 113, E2162–E2171 (2016).
Peters, D. T. et al. Asialoglycoprotein receptor 1 is a specific cell-surface marker for isolating hepatocytes derived from human pluripotent stem cells. Development 143, 1475–1481 (2016).
Aizarani, N. et al. A human liver cell atlas reveals heterogeneity and epithelial progenitors. Nature 572, 199–204 (2019).
Kim, H.-S. et al. Schwann cell precursors from human pluripotent stem cells as a potential therapeutic target for myelin repair. Stem Cell Rep. 8, 1714–1726 (2017).
Liu, Z. et al. Specific marker expression and cell state of Schwann cells during culture in vitro. PLoS ONE 10, e0123278 (2015).
Chan, W. H., Anderson, C. R. & Gonsalvez, D. G. From proliferation to target innervation: signaling molecules that direct sympathetic nervous system development. Cell Tissue Res. 372, 171–193 (2018).
Rohrer, H. Transcriptional control of differentiation and neurogenesis in autonomic ganglia. Eur. J. Neurosci. 34, 1563–1573 (2011).
Chan, W. H. et al. RNA-seq of isolated chromaffin cells highlights the role of sex-linked and imprinted genes in adrenal medulla development. Sci. Rep. 9, 3929 (2019).
Acknowledgements
We thank the patients and their parents for making available the tumor specimens analyzed in this study. We also thank the German Neuroblastoma Biobank for providing these samples. The human embryonic and fetal materials were provided by the joint MRC/Wellcome Trust (grant no. MR/R006237/1) Human Developmental Biology Resource (www.hdbr.org). The institutional review board approved the collection and use of all specimens in this study. We thank J.-P. Mallm and the Single-cell Open Lab, Genomics and Proteomics Core Facility and Light Microscopy Facility at the German Cancer Research Center. The results published in this article are in part based on data generated by the Therapeutically Applicable Research to Generate Effective Treatments (https://ocg.cancer.gov/programs/target) initiative (phs000218). The data used for this analysis are available at https://portal.gdc.cancer.gov/projects. This work was supported by the Fördergesellschaft Kinderkrebs-Neuroblastom-Forschung e.V. (F.W. and S.J.), German-Israeli Helmholtz Research School in Cancer Biology (S.J.), German Ministry of Science and Education (e:Med initiative, grant no. 01ZX1307 to F.W.; grant no. 031L0238 to F.W. and T.H.), EU as part of the EraCoSysMed initiative (Optimize-NB and Infer-NB to F.W. and T. H.), German Cancer Research Center intramural program for interaction projects (Single-cell Open Lab) and DKFZ-Heidelberg Center for Personalized Oncology (HIPO) and National Center for Tumor Disease (NCT 3.0—ENHANCE to F.W.). The laboratory of T.G.P.G. is supported by the Barbara & Wilfried Mohr Foundation.
Author information
Authors and Affiliations
Contributions
S.J. and F.W. designed and coordinated the experiments and data analyses. S.J. performed most of the experiments and data analyses. A.K.S. analyzed the transcription factor activity in the snRNA-seq data. V.K. analyzed the copy number variation in the snRNA-seq data. U.H.T. contributed to the preprocessing of the snRNA-seq and bulk RNA-seq data. E.M.W. performed the RNAscope and western blot experiments. A.G., A.Q., E.C., C.H., A.T. and T.H. contributed to the single-cell data analysis. M.G. performed the ATRA treatment. T.G.P.G. coordinated the immunohistochemical staining. S.J. and F.W. wrote the manuscript with input from K.-O.H. and T.H. F.W. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature Genetics thanks Guoji Guo and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Adrenal gland histology and cell types.
a, b, H&E and immunohistochemical staining of adrenal gland serial sections for CHGA, ALK, ISL1 and KI67. Left panels show overviews over entire glands. Boxes mark regions of insets on the right. Scale bars: left: 1000/500 µm, inset: 200 µm. Sections are derived from 17 pcw (AG07) (a) and 14 pcw (AG08) (b) adrenal glands. Shown are representative results for two out of four samples analyzed in independent experiments.
Extended Data Fig. 2 Adrenal gland cluster, age and sample contribution.
a, Uniform Manifold Approximation and Projection (UMAP) embedding of single cells derived from developing human adrenal glands colored by 45 unique clusters of transcriptionally similar cells. Inset shows the cells colored by cell types as in Fig. 1b. b, c, UMAP embedding from (a) colored by time point of sample collection (b) and individual samples (c). d, Heat map depicting relative expression of marker genes for adrenal gland cell populations as in Fig. 1b. Normalized expression is shown as z-score. e, f, UMAP plot of adrenal medullary cells colored by 16 clusters of transcriptionally similar cells (d) and individual samples (e). Inset shows the cells colored by cell types as in Fig. 1c. g, UMAP visualizations showing adrenal medullary cells from each individual time point integrated using harmony. h, Barplot with fractions of cells in each cell cycle phase in adrenal medullary cell types. i, RNAscope fluorescent RNA in situ hybridization for bridge marker ASCL1, neuroblast marker ISL1 and chromaffin marker TH in human adrenal glands at 8 pcw and 14 pcw. Left panels show composite overviews over entire glands, boxes mark the regions of insets shown on the right. Scale bars: left: 800 µm, inset: 20 µm. Shown are representative results for two out of four samples analyzed in independent experiments.
Extended Data Fig. 3 Adrenal medulla potency and pseudotime.
a, Diffusion map of adrenal medullary cells as shown in Fig. 2a, colored by 19 clusters of transcriptionally similar cells. Start of the pseudotime trajectory is marked with an arrow (cluster 16). b, c, UMAP embedding of adrenal medullary cells without cycling populations colored by 19 clusters of transcriptionally similar cells (b) or cell types (c). Start of the pseudotime trajectory is marked with an arrow (cluster 16). d, f, UMAP plot showing adrenal medullary cells without cycling populations colored by differentiation potential as determined by CytoTRACE (d) and differentiation potency as determined by LandSCENT (f). e, Boxplot with differentiation potencies for adrenal medullary cell populations as determined by LandSCENT. Here, the center line represents the median, upper and lower boundaries the 75th and 25th percentile and whiskers the 1.5x interquartile range. Number of cells: late SCPs: n = 632, SCPs: n = 817, cycling SCPs: n = 288, Bridge: n = 612, con. Prog: n = 1499, Chrom: n = 1346, late Chrom: n = 963, cycling Nbs: n = 1064, Nbs: n = 1388, late Nbs: n = 2130. g, Diffusion map of adrenal medullary cells colored by SCP-to-late SCP pseudotime trajectory as determined by slingshot. h, UMAP visualization illustrating adrenal medullary cells without cycling populations colored by pseudotime as determined by monocle3. Start of the pseudotime trajectory is marked with an arrow. i, Gene expression of selected genes over pseudotime in chromaffin cell and neuroblast trajectories.
Extended Data Fig. 4 Neuroblastoma cluster, sample and cell type contribution.
a, UMAP visualization illustrating 64.769 single neuroblastoma cells integrated using harmony and colored by cell types. b, Heat map showing expression of canonical cell type markers in cells derived from neuroblastoma tumors. Color indicates relative expression as z-score. c, UMAP embedding showing single neuroblastoma cells integrated using harmony and colored by inferred copy number status. d, Barplot with annotated cell type fractions for each neuroblastoma tumor. e, Boxplots showing expression of published adrenergic and mesenchymal neuroblastoma signatures in single neuroblastoma cells grouped by subgroup. Here, the center line represents the median, upper and lower boundaries the 75th and 25th percentile and whiskers the 1.5x interquartile range. Number of cells: LR: n = 21,086, ALT/TERT: n = 12,719, MES: n = 8980, MYCN: n = 16,527. f, g, UMAP plot showing neuroblastoma cells integrated using harmony and colored by clusters of transcriptionally similar cells (f) and sample (g).
Extended Data Fig. 5 Neuroblastoma copy number variations.
a–n Inferred copy number profiles of neuroblastoma cells using InferCNV with normal neuroblasts as reference. Below: Copy number profiles of the same tumors derived from whole genome sequencing data.
Extended Data Fig. 6 Similarity of single neuroblastoma cells to normal developing adrenal medullary cell populations.
a–l, Heat maps with similarity scores of single neuroblastoma cells and fetal adrenal medullary cell populations for neuroblastoma tumors not shown in Fig. 3. m, Bar plots illustrating proportion of neuroblastoma cells assigned to adrenal medullary cells or cells from published fetal and adult brain data sets by neuroblastoma subgroup. Cells are assigned to the normal population with highest similarity score.
Extended Data Fig. 7 Similarity of single cells from neuroblastoma cell lines to normal developing adrenal medullary cell populations.
a–c, UMAP visualization of single SK-N-SH cells colored by adrenergic, mesenchymal and intermediate population (a), published mesenchymal neuroblastoma signature expression (b) or adrenergic neuroblastoma signature expression (c). d, Heat map with similarity scores of single SK-N-SH cells to fetal adrenal medullary cell populations. Top bar indicates SK-N-SH subpopulation identity. e, Bar plots illustrating proportion of SK-N-SH cells assigned to each normal adrenal medullary cell population. Cells are assigned to the normal population with highest similarity score. f-h, UMAP embedding of single SK-N-AS cells colored by clusters of transcriptionally similary cells (f), published mesenchymal neuroblastoma signature expression (g) or adrenergic neuroblastoma signature expression (h). i, Heat map with similarity scores of single SK-N-AS cells to fetal adrenal medullary cell populations. j, Bar plots illustrating proportion of SK-N-AS cells assigned to each normal adrenal medullary cell population. Cells are assigned to the normal population with highest similarity score.
Extended Data Fig. 9 Regulation of neuroblastoma by markers of normal adrenal medullary development.
a, Dot plot showing expression of genes with strong enhancers described to engage in enhancer hijacking events in neuroblastoma, in adrenal medullary cell populations. b, Violin plots with normalized expression of adrenal medullary developmental genes in neuroblastoma subgroups. c, Heat map illustrating activity of adrenal medullary transcription factors in neuroblastoma shown as fraction of cells in which the regulon was found active by subgroup. d, UMAP embedding showing single MYCN-amplified IMR5/75 neuroblastoma cells with tetracyclin-inducible shRNAs targeting MYCN and generating MYCN high (-Tet) and MYCN low (+Tet) cells. Cells are colored by levels of regulatable MYCN. e, UMAP visualization of MYCN low and MYCN high cells colored by cell cycle phase. f, Barplot with fractions of cells in each cell cycle phase in MYCN high and MYCN low cells. g, MYCN protein levels in MYCN high and MYCN low IMR5/75 neuroblastoma cells. Detection by western blot using vinculin as loading control. Analysis was performed once on cells from the same experiment as used for single-cell sequencing. h, Violin plots show expression of signatures for Bridge (p < 2.22 × 10−16), con. Progenitor cells (p < 2.22 × 10−16), Chromaffin cells (p = 0.034), late Chromaffin cells (p < 2.22 × 10−16), SCPs (p < 2.22 × 10−16) and late SCPs (p < 2.22 × 10−16) in MYCN-amplified (MYCN high) neuroblastoma cells and MYCN-amplified cells upon MYCN knockdown (MYCN low). Statistical significance was determined by two-sided Wilcoxon rank-sum test.
Extended Data Fig. 10 Association of neuroblastoma cell type composition with clinical parameters.
a, Hierarchical clustering of patient samples based on abundance of fetal adrenal medullary cell populations without cycling populations determined by deconvolution with BSEQ-sc (SEQC cohort). b, Composition of neuroblastoma tumors by subgroup based on deconvolution of bulk RNA-seq data with fetal adrenal cell populations without cycling populations using BSEQ-sc (SEQC cohort). c, Hierarchical clustering of patient samples based on abundance of fetal adrenal medullary cell populations determined by BSEQ-sc (TARGET cohort). d-i, Kaplan-Meier analysis of event-free (d, f, h) or overall (e, g, i) survival in neuroblastoma patients according to the relative abundance of late neuroblasts (d–g), neuroblasts (h) or cycling neuroblasts (i) determined by deconvolution of tumors with fetal adrenal cell populations. Panels f and g show deconvolution results for the TARGET cohort, panels e and i show deconvolution results for the SEQC cohort and panels d and h show deconvolution results for the SEQC cohort when excluding cycling cells from the analysis. P-values were calculated using the two-sided log-rank test.
Supplementary information
Supplementary Tables
Supplementary Tables 1–11.
Source data
Source Data Fig. 1
Unprocessed western blots.
Rights and permissions
About this article
Cite this article
Jansky, S., Sharma, A.K., Körber, V. et al. Single-cell transcriptomic analyses provide insights into the developmental origins of neuroblastoma. Nat Genet 53, 683–693 (2021). https://doi.org/10.1038/s41588-021-00806-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41588-021-00806-1
This article is cited by
-
Developmental origins shape the paediatric cancer genome
Nature Reviews Cancer (2024)
-
MUW researcher of the month
Wiener klinische Wochenschrift (2024)
-
Integrative analysis with machine learning identifies diagnostic and prognostic signatures in neuroblastoma based on differentially DNA methylated enhancers between INSS stage 4 and 4S neuroblastoma
Journal of Cancer Research and Clinical Oncology (2024)
-
A human neural crest model reveals the developmental impact of neuroblastoma-associated chromosomal aberrations
Nature Communications (2024)
-
Effective methods for bulk RNA-seq deconvolution using scnRNA-seq transcriptomes
Genome Biology (2023)