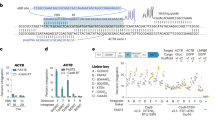
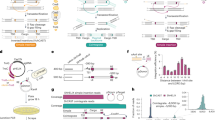
Abstract
Conventional genome engineering with CRISPR–Cas9 creates double-strand breaks (DSBs) that lead to undesirable byproducts and reduce product purity. Here we report an approach for programmable integration of large DNA sequences in human cells that avoids the generation of DSBs by using Type I-F CRISPR-associated transposases (CASTs). We optimized DNA targeting by the QCascade complex through protein design and developed potent transcriptional activators by exploiting the multi-valent recruitment of the AAA+ ATPase TnsC to genomic sites targeted by QCascade. After initial detection of plasmid-based integration, we screened 15 additional CAST systems from a wide range of bacterial hosts, identified a homolog from Pseudoalteromonas that exhibits improved activity and further increased integration efficiencies. Finally, we discovered that bacterial ClpX enhances genomic integration by multiple orders of magnitude, likely by promoting active disassembly of the post-integration CAST complex, akin to its known role in Mu transposition. Our work highlights the ability to reconstitute complex, multi-component machineries in human cells and establishes a strong foundation to exploit CRISPR-associated transposases for eukaryotic genome engineering.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Sequencing data have been deposited in the National Center for Biotechnology Informationʼs Sequence Read Archive under Gene Expression Omnibus accession number GSE223174 (ref. 92). Source data are provided with this paper.
References
Pickar-Oliver, A. & Gersbach, C. A. The next generation of CRISPR–Cas technologies and applications. Nat. Rev. Mol. Cell Biol. 20, 490–507 (2019).
Knott, G. J. & Doudna, J. A. CRISPR–Cas guides the future of genetic engineering. Science 361, 866–869 (2018).
Anzalone, A. V., Koblan, L. W. & Liu, D. R. Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 38, 824–844 (2020).
Maruyama, T. et al. Inhibition of non-homologous end joining increases the efficiency of CRISPR/Cas9-mediated precise genome editing. Nature 33, 538–542 (2015).
Nakade, S. et al. Microhomology-mediated end-joining-dependent integration of donor DNA in cells and animals using TALENs and CRISPR/Cas9. Nat. Commun. 5, 5560 (2014).
Chu, V. T. et al. Increasing the efficiency of homology-directed repair for CRISPR–Cas9-induced precise gene editing in mammalian cells. Nat. Biotechnol. 33, 543–548 (2015).
Yeh, C. D., Richardson, C. D. & Corn, J. E. Advances in genome editing through control of DNA repair pathways. Nat. Cell Biol. 21, 1468–1478 (2019).
Heyer, W.-D., Ehmesn, K. T. & Liu, J. Regulation of homologous recombination in eukaryotes. Annu. Rev. Genet. 44, 113–139 (2010).
Moynahan, M. E. & Jasin, M. Mitotic homologous recombination maintains genomic stability and suppresses tumorigenesis. Nat. Rev. Mol. Cell Biol. 11, 196–207 (2010).
Lin, S., Staahl, B. T., Alla, R. K. & Doudna, J. A. Enhanced homology-directed human genome engineering by controlled timing of CRISPR/Cas9 delivery. eLife 3, e04766 (2014).
Zuccaro, M. V. et al. Allele-specific chromosome removal after Cas9 cleavage in human embryos. Cell 183, 1650–1654 (2020).
Adikusuma, F. et al. Large deletions induced by Cas9 cleavage. Nature 560, E8–E9 (2018).
Kosicki, M., Tomberg, K. & Bradley, A. Repair of double-strand breaks induced by CRISPR–Cas9 leads to large deletions and complex rearrangements. Nat. Biotechnol. 36, 765–771 (2018).
Leibowitz, M. L. et al. Chromothripsis as an on-target consequence of CRISPR–Cas9 genome editing. Nat. Genet. 53, 895–905 (2021).
Kim, Y. B. et al. Increasing the genome-targeting scope and precision of base editing with engineered Cas9-cytidine deaminase fusions. Nat. Biotechnol. 35, 371–376 (2017).
Gaudelli, N. M. et al. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 551, 464–471 (2017).
Anzalone, A. V. et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157 (2019).
Anzalone, A. V. et al. Programmable deletion, replacement, integration and inversion of large DNA sequences with twin prime editing. Nat. Biotechnol. 40, 731–740 (2021).
Yarnall, M. T. N. et al. Drag-and-drop genome insertion of large sequences without double-strand DNA cleavage using CRISPR-directed integrases. Nat. Biotechnol. https://doi.org/10.1038/s41587-022-01527-4 (2022).
Naldini, L., Trono, D. & Verma, I. M. Lentiviral vectors, two decades later. Science 353, 1101–1102 (2016).
Querques, I. et al. A highly soluble Sleeping Beauty transposase improves control of gene insertion. Nat. Biotechnol. 37, 1502–1512 (2019).
Yusa, K., Zhou, L., Li, M. A., Bradley, A. & Craig, N. L. A hyperactive piggyBac transposase for mammalian applications. Proc. Natl Acad. Sci. USA 108, 1531–1536 (2011).
Tipanee, J., Vandendriessche, T. & Chuah, M. K. Transposons: moving forward from preclinical studies to clinical trials. Hum. Gene Ther. 28, 1087–1104 (2017).
Gaidukov, L. et al. A multi-landing pad DNA integration platform for mammalian cell engineering. Nucleic Acids Res. 46, 4072–4086 (2018).
Durrant, M. G. et al. Systematic discovery of recombinases for efficient integration of large DNA sequences into the human genome. Nat. Biotechnol. https://doi.org/10.1038/s41587-022-01494-w (2022).
Hew, B. E., Sato, R., Mauro, D., Stoytchev, I. & Owens, J. B. RNA-guided piggyBac transposition in human cells. Synth. Biol. 4, ysz018 (2019).
Kovač, A. et al. RNA-guided retargeting of Sleeping Beauty transposition in human cells. eLife 9, e53868 (2020).
Luo, W. et al. Comparative analysis of chimeric ZFP-, TALE- and Cas9-piggyBac transposases for integration into a single locus in human cells. Nucleic Acids Res. 45, 8411–8422 (2017).
Chen, S. P. & Wang, H. H. An engineered Cas-Transposon system for programmable and site-directed DNA transpositions. CRISPR J. 2, 376–394 (2019).
Klompe, S. E., Vo, P. L. H., Halpin-Healy, T. S. & Sternberg, S. H. Transposon-encoded CRISPR–Cas systems direct RNA-guided DNA integration. Nature 571, 219–225 (2019).
Vo, P. L. H. et al. CRISPR RNA-guided integrases for high-efficiency, multiplexed bacterial genome engineering. Nat. Biotechnol. 39, 480–489 (2021).
Klompe, S. E. et al. Evolutionary and mechanistic diversity of type I-F CRISPR-associated transposons. Mol. Cell 82, 616–628 (2022).
Cameron, P. et al. Harnessing type I CRISPR–Cas systems for genome engineering in human cells. Nat. Biotechnol. 37, 1471–1477 (2019).
Chen, Y. et al. Repurposing type I-F CRISPR–Cas system as a transcriptional activation tool in human cells. Nat. Commun. 11, 3136 (2020).
Pickar-Oliver, A. et al. Targeted transcriptional modulation with type I CRISPR–Cas systems in human cells. Nat. Biotechnol. 37, 1493–1501 (2019).
Dolan, A. E. et al. Introducing a spectrum of long-range genomic deletions in human embryonic stem cells using type I CRISPR–Cas. Mol. Cell 74, 936–950 (2019).
Young, J. K. et al. The repurposing of type I-E CRISPR-Cascade for gene activation in plants. Commun. Biol. 2, 383 (2019).
Strecker, J. et al. RNA-guided DNA insertion with CRISPR-associated transposases. Science 364, 48–53 (2019).
Saito, M. et al. Dual modes of CRISPR-associated transposon homing. Cell 184, 2441–2453.e18 (2021).
Vo, P. L. H., Acree, C., Smith, M. L. & Sternberg, S. H. Unbiased profiling of CRISPR RNA-guided transposition products by long-read sequencing. Mob. DNA 12, 13 (2021).
Halpin-Healy, T. S., Klompe, S. E., Sternberg, S. H. & Fernández, I. S. Structural basis of DNA targeting by a transposon-encoded CRISPR–Cas system. Nature 577, 271–274 (2020).
Peters, J. E. Tn7. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.MDNA3-0010-2014 (2014).
Hoffmann, F. T. et al. Selective TnsC recruitment enhances the fidelity of RNA-guided transposition. Nature 609, 384–393 (2022).
Behler, J. & Hess, W. R. Approaches to study CRISPR RNA biogenesis and the key players involved. Methods 172, 12–26 (2020).
Szczelkun, M. D., Tikhomirova, M. S., Sinkunas, T., Gasiunas, G. & Karvelis, T. Direct observation of R-loop formation by single RNA-guided Cas9 and Cascade effector complexes. Proc. Natl Acad. Sci. USA 111, 9798–9803 (2014).
Gilbert, L. A. et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154, 442–451 (2013).
Mali, P. et al. CAS9 transcriptional activators for target specificity screening and paired nickases for cooperative genome engineering. Nat. Biotechnol. 31, 833–838 (2013).
Perez-pinera, P. et al. RNA-guided gene activation by CRISPR–Cas9-based transcription factors. Nat. Methods 10, 973–976 (2013).
Tanenbaum, M. E., Gilbert, L. A., Qi, L. S., Weissman, J. S. & Vale, R. D. A protein-tagging system for signal amplification in gene expression and fluorescence imaging. Cell 159, 635–646 (2014).
Konermann, S. et al. Genome-scale transcriptional activation by an engineered CRISPR–Cas9 complex. Nature 517, 583–588 (2015).
Park, J. U. et al. Structural basis for target site selection in RNA-guided DNA transposition systems. Science 373, 768–774 (2021).
Querques, I., Schmitz, M., Oberli, S., Chanez, C. & Jinek, M. Target site selection and remodelling by type V CRISPR-transposon systems. Nature 599, 49–502 (2021).
Chavez, A. et al. Highly-efficient Cas9-mediated transcriptional programming. Nat. Methods 12, 326–3228 (2015).
Thakore, P. I. et al. Highly specific epigenome editing by CRISPR–Cas9 repressors for silencing of distal regulatory elements. Nat. Methods 12, 1143–1149 (2015).
Wang, T., Larcher, L. M., Ma, L. & Veedu, R. N. Systematic screening of commonly used commercial transfection reagents towards efficient transfection of single-stranded oligonucleotides. Molecules 23, 2564 (2018).
Walker, M. W. G., Klompe, S. E., Zhang, D. J. & Sternberg, S. H. Transposon mutagenesis libraries reveal novel molecular requirements during CRISPR RNA-guided DNA integration. Preprint at https://www.biorxiv.org/content/10.1101/2023.01.19.524723v1 (2023).
Sarnovsky, R. J., May, E. W. & Craig, N. L. The Tn7 transposase is a heteromeric complex in which DNA breakage and joining activities are distributed between different gene products. EMBO J. 15, 6348–6361 (1996).
North, S. H. & Nakai, H. Host factors that promote transpososome disassembly and the PriA-PriC pathway for restart primosome assembly. Mol. Microbiol. 56, 1601–1616 (2005).
Adeyemi, R. O. et al. The Protexin complex counters resection on stalled forks to promote homologous recombination and crosslink repair. Mol. Cell 81, 4440–4456 (2021).
Ciccia, A. & Elledge, S. J. The DNA damage response: making it safe to play with knives. Mol. Cell 40, 179–204 (2010).
Holder, J. W. & Craig, N. L. Architecture of the Tn7 posttransposition complex: an elaborate nucleoprotein structure. J. Mol. Biol. 401, 167–181 (2010).
Levchenko, I., Luo, L. & Baker, T. A. Disassembly of the Mu transposase tetramer by the ClpX chaperone. Genes Dev. 9, 2399–2408 (1995).
Kruklitis, R., Welty, D. J. & Nakai, H. ClpX protein of Escherichia coli activates bacteriophage Mu transposase in the strand transfer complex for initiation of Mu DNA synthesis. EMBO J. 15, 935–944 (1996).
Mhammedi-Alaoul, A., Pato, M. & Gama, M.-J. & Toussaint, A. A new component of bacteriophage Mu replicative transposition machinery: the Escherichia coli ClpX protein. Mol. Microbiol. 11, 1109–1116 (1994).
Abdelhakim, A. H., Oakes, E. C., Sauer, R. T. & Baker, T. A. Unique contacts direct high-priority recognition of the tetrameric Mu transposase-DNA complex by the AAA+ unfoldase ClpX. Mol. Cell 30, 39–50 (2008).
Sauer, R. T. & Baker, T. A. AAA+ proteases: ATP-fueled machines of protein destruction. Annu. Rev. Biochem. 80, 587–612 (2011).
Levchenko, I., Yamauchi, M. & Baker, T. A. ClpX and MuB interact with overlapping regions of Mu transposase: implications for control of the transposition pathway. Genes Dev. 11, 1561–1572 (1997).
Baker, T. A. & Sauer, R. T. ClpXP, an ATP-powered unfolding and protein-degradation machine. Biochim. Biophys. Acta Mol. Cell Res. 1823, 15–28 (2012).
Hersch, G. L., Burton, R. E., Bolon, D. N., Baker, T. A. & Sauer, R. T. Asymmetric interactions of ATP with the AAA+ ClpX6 unfoldase: allosteric control of a protein machine. Cell 121, 1017–1027 (2005).
Joshi, S. A., Hersch, G. L., Baker, T. A. & Sauer, R. T. Communication between ClpX and ClpP during substrate processing and degradation. Nat. Struct. Mol. Biol. 11, 404–411 (2004).
Siddiqui, S. M., Sauer, R. T. & Baker, T. A. Role of the processing pore of the ClpX AAA+ ATPase in the recognition and engagement of specific protein substrates. Genes Dev. 18, 369–374 (2004).
Strecker, J., Zhang, F. & Ladha, A. CRISPR-associated transposase systems and methods of use thereof. https://patents.google.com/patent/WO2020131862A1/en (2020).
Tou, C. J., Orr, B. & Kleinstiver, B. P. Precise cut-and-paste DNA insertion using engineered type V-K CRISPR-associated transposases. Nat. Biotechnol. https://doi.org/10.1038/s41587-022-01574-x (2023).
Özcan, A. et al. Programmable RNA targeting with the single-protein CRISPR effector Cas7-11. Nature 597, 720–725 (2021).
Shen, Y. et al. Structural basis for DNA targeting by the Tn7 transposon. Nat. Struct. Mol. Biol. 29, 143–151 (2022).
Gu, B. et al. Transcription-coupled changes in nuclear mobility of mammalian cis-regulatory elements. Science 359, 1050–1055 (2018).
Chen, B., Guan, J. & Huang, B. Imaging specific genomic DNA in living cells. Annu. Rev. Biophys. 45, 1–23 (2016).
Schmitz, M., Querques, I., Oberli, S., Chanez, C. & Jinek, M. Structural basis for the assembly of the type V CRISPR-associated transposon complex. Cell 185, 4999–5010 (2022).
Fricke, T. et al. Targeted RNA knockdown by a type III CRISPR–Cas complex in zebrafish. CRISPR J. 3, 299–313 (2020).
Petassi, M. T., Hsieh, S. & Peters, J. E. Guide RNA categorization enables target site choice in Tn7-CRISPR-Cas transposons. Cell 183, 1757–1771 (2020).
Yeo, N. C. et al. An enhanced CRISPR repressor for targeted mammalian gene regulation. Nat. Methods 15, 611–616 (2018).
Lee, T. I., Johnstone, S. E. & Young, R. A. Chromatin immunoprecipitation and microarray-based analysis of protein location. Nat. Protoc. 1, 729–748 (2006).
Weinberg, D. N. et al. The histone mark H3K36me2 recruits DNMT3A and shapes the intergenic DNA methylation landscape. Nature 573, 281–286 (2019).
Qiu, X. et al. CoBRA: Containerized Bioinformatics Workflow for Reproducible ChIP/ATAC-seq Analysis. Genomics Proteomics Bioinformatics 19, 652–661 (2021).
Li, H. et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics 25, 2078–2079 (2009).
Zhang, Y. et al. Model-based analysis of ChIP-Seq (MACS). Genome Biol. 9, R137 (2008).
Robinson, J. T. et al. Integrative genomics viewer. Nat. Biotechnol. 29, 24–26 (2011).
Quinlan, A. R. & Hall, I. M. BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26, 841–842 (2010).
Amemiya, H. M., Kundaje, A. & Boyle, A. P. The ENCODE blacklist: identification of problematic regions of the genome. Sci. Rep. 9, 9354 (2019).
Stark, R. & Brown, G. DiffBind: differential binding analysis of ChIP-Seq peak data. http://bioconductor.org/packages/release/bioc/vignettes/DiffBind/inst/doc/DiffBind.pdf 1–29 (2011).
Ramírez, F. et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 44, W160–W165 (2016).
Lampe, G. D. et al. Integration in human cells without double-strand breaks using CRISPR RNA-guided transposases. Gene Expression Omnibus https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE223174 (2023).
Acknowledgements
We thank N. Jaber and S. R. Pesari for laboratory support, L. F. Landweber for qPCR instrument access, A. Santiago-Frangos for helpful ClpX discussions, the Columbia University Institute for Genomic Medicine for ddPCR instrument access, W. Frankel for thermocycler instrument access, the Columbia Stem Cell Initiative Flow Cytometry Core, M. Kissner and R. Gordon-Schneider for assistance in cell sorting and flow cytometry analysis, and the JP Sulzberger Columbia Genome Center for NGS support. We thank C. Lu and V. Sahu for cell sonicator instrument access and valuable discussions on ChIP-seq analysis. R.T.K. is supported by NIH grant 1F31HL167530-01 from the National Heart, Lung, and Blood Institute. A.C. is supported by a Career Award for Medical Scientists from the Burroughs Wellcome Fund and NIH grant HG011855-01 from the National Human Genome Research Institute. S.H.S. is supported by NIH grant DP2HG011650, a Pew Biomedical Scholarship, a Sloan Research Fellowship, an Irma T. Hirschl Career Scientist Award, and a generous startup package from the Columbia University Irving Medical Center Dean’s Office and the Vagelos Precision Medicine Fund.
Author information
Authors and Affiliations
Contributions
G.D.L. performed western blots, plasmid-based reporter assays and experiments screening CAST homologs and host proteins. R.T.K. performed genomic activation assays, ChIP experiments and ddPCR analyses. G.D.L. and R.T.K. performed integration assays and quantified integration efficiency by qPCR and amplicon sequencing. T.S.H.-H. performed initial human cell experiments and, together with M.I.H., tested tagged constructs for activity in E. coli. S.E.K. tested tagged constructs in E. coli and assisted with initial NGS library preparation and data analysis. P.L.H.V. designed and cloned constructs for initial I-E activation assays. S.T. performed ChIP-seq analyses. A.C. provided expert support and helped S.H.S. supervise the project. S.H.S., G.D.L., R.T.K. and A.C. discussed the data and wrote the manuscript, with input from all authors.
Corresponding author
Ethics declarations
Competing interests
Columbia University has filed a patent application related to this work. S.E.K., P.L.H.V., A.C. and S.H.S. are inventors on other patents and patent applications related to CRISPR–Cas systems and uses thereof. A.C. is a consultant for Vor Biopharma and an equity holder and scientific advisor for Cellgorithmics. S.H.S. is a co-founder of and scientific advisor to Dahlia Biosciences, a scientific advisor to Prime Medicine and CrisprBits and an equity holder in Dahlia Biosciences and CrisprBits. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature Biotechnology thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Figs. 1–15.
Supplementary Tables 1–4
Supplementary Tables.
Source data
Source Data Fig. 1
Unprocessed western blots.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lampe, G.D., King, R.T., Halpin-Healy, T.S. et al. Targeted DNA integration in human cells without double-strand breaks using CRISPR-associated transposases. Nat Biotechnol 42, 87–98 (2024). https://doi.org/10.1038/s41587-023-01748-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-023-01748-1