Abstract
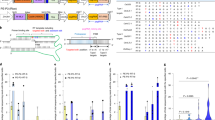
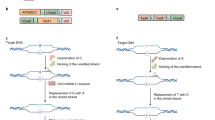
Prime editors, which are CRISPR–Cas9 nickase (H840A)–reverse transcriptase fusions programmed with prime editing guide RNAs (pegRNAs), can edit bases in mammalian cells without donor DNA or double-strand breaks. We adapted prime editors for use in plants through codon, promoter, and editing-condition optimization. The resulting suite of plant prime editors enable point mutations, insertions and deletions in rice and wheat protoplasts. Regenerated prime-edited rice plants were obtained at frequencies of up to 21.8%.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Data availability
All data supporting the findings of this study are available in the article or in Supplementary information files, or are available from the corresponding author upon request. In terms of sequence data, the rice LOC_Os identifiers (http://rice.plantbiology.msu.edu/) are LOC_Os01g55540 (OsAAT), LOC_Os03g54790 (OsALS), LOC_Os03g05730 (OsCDC48), LOC_Os09g26999 (OsDEP1), LOC_Os06g04280 (OsEPSPS), LOC_Os08g03290 (OsGAPDH). The NCBI GenBank identifiers are KJ697755 (TaGW2), KF009556 (TaMLO), KJ000052 (TaGASR7), JF683316 (TaDME), GU167921 (TaLOX2), FJ459808 (TaUbi10). The deep sequencing data have been deposited in an NCBI BioProject database (accession codes PRJNA605069 and PRJNA605074). Plasmids encoding nCas9-PPE, QPM-sgR (for nicking sgRNA construction), and pH-nCas9-PPE (for pH-nCas9-PPE2/3/3b construction) will be made available through Addgene.
References
Voytas, D. F. & Gao, C. Precision genome engineering and agriculture: opportunities and regulatory challenges. PLoS Biol. 12, e1001877 (2014).
Wang, W. et al. Genomic variation in 3,010 diverse accessions of Asian cultivated rice. Nature 557, 43–49 (2018).
Puchta, H. & Fauser, F. Gene targeting in plants: 25 years later. Int. J. Dev. Biol. 57, 629–637 (2013).
Chen, K., Wang, Y., Zhang, R., Zhang, H. & Gao, C. CRISPR/Cas genome editing and precision plant breeding in agriculture. Annu. Rev. Plant Biol. 29, 667–697 (2019).
Svitashev, S. et al. Targeted mutagenesis, precise gene editing, and site-specific gene insertion in maize using Cas9 and guide RNA. Plant Physiol. 169, 931–945 (2015).
Ran, Y., Liang, Z. & Gao, C. Current and future editing reagent delivery systems for plant genome editing. Sci. China Life Sci. 60, 490–505 (2017).
Komor, A. C., Kim, Y. B., Packer, M. S., Zuris, J. A. & Liu, D. R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424 (2016).
Nishida, K. et al. Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science 353, 1248–1256 (2016).
Gaudelli, N. M. et al. Programmable base editing of A•T to G•C in genomic DNA without DNA cleavage. Nature 551, 464–471 (2017).
Kim, Y. B. et al. Increasing the genome-targeting scope and precision of base editing with engineered Cas9-cytidine deaminase fusions. Nat. Biotechnol. 35, 371–376 (2017).
Anzalone, A. V. et al. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157 (2019).
Plant, A. L., Covey, S. N. & Grierson, D. Detection of a subgenomic mRNA for gene V, the putative reverse transcriptase gene of cauliflower mosaic virus. Nucleic Acids Res. 13, 8305–8321 (1985).
Lim, D. & Maas, W. K. Reverse transcriptase-dependent synthesis of a covalently linked, branched DNA-RNA compound in E. coli B. Cell 56, 891–904 (1989).
Zong, Y. et al. Precise base editing in rice, wheat and maize with a Cas9-cytidine deaminase fusion. Nat. Biotechnol. 35, 438–440 (2017).
Gao, Y. & Zhao, Y. Self-processing of ribozyme-flanked RNAs into guide RNAs in vitro and in vivo for CRISPR-mediated genome editing. J. Integr. Plant Biol. 56, 343–349 (2014).
Li, C. et al. Expanded base editing in rice and wheat using a Cas9-adenosine deaminase fusion. Genome Biol. 19, 59 (2018).
Shan, Q. et al. Targeted genome modification of crop plants using a CRISPR-Cas system. Nat. Biotechnol. 31, 686–688 (2013).
Wang, Y. et al. Simultaneous editing of three homoeoalleles in hexaploid bread wheat confers heritable resistance to powdery mildew. Nat. Biotechnol. 32, 947–951 (2014).
Xing, H. et al. A CRISPR/Cas9 toolkit for multiplex genome editing in plants. BMC Plant Biol. 14, 327 (2014).
Čermák, T. et al. A multipurpose toolkit to enable advanced genome engineering in plants. Plant Cell 29, 1196–1217 (2017).
Zong, Y. et al. Efficient C-to-T base editing in plants using a fusion of nCas9 and human APOBEC3A. Nat. Biotechnol. 36, 950–953 (2018).
Shan, Q. et al. Rapid and efficient gene modification in rice and Brachypodium using TALENs. Mol. Plant 6, 1365–1368 (2013).
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (31788103), the Strategic Priority Research Program of the Chinese Academy of Sciences (Precision Seed Design and Breeding, XDA24000000), and the National Key Research and Development Program of China (2016YFD0101804). A.V.A, A.R., J.L.D., and D.R.L. were supported by US National Institutes of Health (NIH) grants U01AI142756, RM1HG009490, R01EB022376, R35GM118062, and by the Howard Hughes Medical Institute (HHMI).
Author information
Authors and Affiliations
Contributions
Q.L., Y.Z., C.X., Y.W., A.V.A., A.R, J.L.D, D.R.L., and C.G. designed the project; Q.L., Y.Z., C.X., S.W., S.J., and Z.Z. performed the experiments; Q.L., A.V.A., A.R, J.L.D, D.R.L., and C.G. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have submitted a patent application based on the results reported in this paper. D.R.L. is a consultant for and co-founder of Beam Therapeutics, Prime Medicine, Pairwise Plants, and Editas Medicine—companies that use genome editing.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Figures
Supplementary Figures 1–8, Supplementary Tables 1–7, Supplementary Sequences 1–3, and Supplementary Data 1.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lin, Q., Zong, Y., Xue, C. et al. Prime genome editing in rice and wheat. Nat Biotechnol 38, 582–585 (2020). https://doi.org/10.1038/s41587-020-0455-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-020-0455-x
This article is cited by
-
Application of genome editing techniques to regulate gene expression in crops
BMC Plant Biology (2024)
-
BacPE: a versatile prime-editing platform in bacteria by inhibiting DNA exonucleases
Nature Communications (2024)
-
Precise genome-editing in human diseases: mechanisms, strategies and applications
Signal Transduction and Targeted Therapy (2024)
-
Microbiome homeostasis on rice leaves is regulated by a precursor molecule of lignin biosynthesis
Nature Communications (2024)
-
Precise integration of large DNA sequences in plant genomes using PrimeRoot editors
Nature Biotechnology (2024)