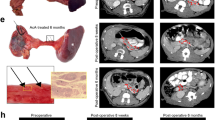
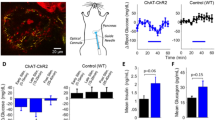
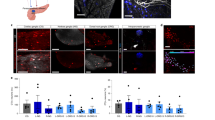
Abstract
Vagus nerve stimulation can ameliorate autoimmune diseases such as rheumatoid arthritis by modulation of the immune system. Its efficacy for the treatment of type 1 diabetes has not been explored, in part because the nerves projecting to the pancreatic lymph nodes (pLNs) in mice are unmapped. Here, we map the nerve projecting to the pancreas and pLNs in mice and use a minimally invasive surgical procedure to implant micro-cuff electrodes onto the nerve. Pancreatic nerve electrical stimulation (PNES) resulted in β-adrenergic receptor-mediated-accumulation of B and T cells in pLNs and reduced production of pro-inflammatory cytokines following lipopolysaccharide stimulation. Autoreactive T cells showed reduced proliferation in pLNs of mice receiving PNES as compared to sham controls. In a spontaneous mouse model of autoimmune diabetes, PNES inhibited disease progression in diabetic mice.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Datasets that support the findings of this study are available from the corresponding author upon reasonable request.
Change history
19 February 2020
In the supplementary information originally posted for this article, characters were corrupted in the figure lettering. The error has been corrected online.
References
Padro, C. J. & Sanders, V. M. Neuroendocrine regulation of inflammation. Semin. Immunol.26, 357–368 (2014).
Koopman, F. A. et al. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc. Natl Acad. Sci. USA113, 8284–8289 (2016).
Bonaz, B. et al. Chronic vagus nerve stimulation in Crohn’s disease: a 6-month follow-up pilot study. Neurogastroenterol. Motil.28, 948–953 (2016).
Herold, K. C. Restoring immune balance in type 1 diabetes. Lancet Diabetes Endocrinol.1, 261–263 (2013).
Coppieters, K. & von Herrath, M. The development of immunotherapy strategies for the treatment of type 1 diabetes. Front. Med.5, 283 (2018).
Duclaux, R., Mei, N. & Ranieri, F. Conduction velocity along the afferent vagal dendrites: a new type of fibre. J. Physiol.260, 487–495 (1976).
Nakai, A., Hayano, Y., Furuta, F., Noda, M. & Suzuki, K. Control of lymphocyte egress from lymph nodes through β2-adrenergic receptors. J. Exp. Med.211, 2583–2598 (2014).
Faustman, D. L. et al. TNF, TNF inducers, and TNFR2 agonists: a new path to type 1 diabetes treatment. Diabetes Metab. Res. Rev. https://doi.org/10.1002/dmrr.2941 (2018).
Mandrup-Poulsen, T. Interleukin-1 antagonists and other cytokine blockade strategies for type 1 diabetes. Rev. Diabet. Stud. RDS9, 338–347 (2012).
Chen, Y.-L. et al. Correlation between serum interleukin-6 level and type 1 diabetes mellitus: a systematic review and meta-analysis. Cytokine94, 14–20 (2017).
Kurts, C., Heath, W. R., Kosaka, H., Miller, J. F. & Carbone, F. R. The peripheral deletion of autoreactive CD8+ T cells induced by cross-presentation of self-antigens involves signaling through CD95 (Fas, Apo-1). J. Exp. Med.188, 415–420 (1998).
Amrani, A. et al. Perforin-independent beta-cell destruction by diabetogenic CD8(+) T lymphocytes in transgenic nonobese diabetic mice. J. Clin. Invest.103, 1201–1209 (1999).
Christianson, S. W., Shultz, L. D. & Leiter, E. H. Adoptive transfer of diabetes into immunodeficient NOD-scid/scid mice. Relative contributions of CD4+ and CD8+ T-cells from diabetic versus prediabetic NOD.NON-Thy-1a donors. Diabetes42, 44–55 (1993).
Hervé, J. et al. β2-Adrenoreceptor agonist inhibits antigen cross-presentation by dendritic cells. J. Immunol.190, 3163–3171 (2013).
Hogquist, K. A. et al. T cell receptor antagonist peptides induce positive selection. Cell76, 17–27 (1994).
Kurts, C., Miller, J. F., Subramaniam, R. M., Carbone, F. R. & Heath, W. R. Major histocompatibility complex class I-restricted cross-presentation is biased towards high dose antigens and those released during cellular destruction. J. Exp. Med.188, 409–414 (1998).
Madisen, L. et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat. Neurosci.13, 133–140 (2010).
Savitt, J. M., Jang, S. S., Mu, W., Dawson, V. L. & Dawson, T. M. Bcl-x is required for proper development of the mouse substantia nigra. J. Neurosci.25, 6721–6728 (2005).
Acknowledgements
This work was funded by a collaborative research grant from GlaxoSmithKline Bioelectronics R&D to P.B. This work was also supported by the LABEX SIGNALIFE (no. ANR-11-LABX-0028-01) and the FHU Oncoage.
Author information
Authors and Affiliations
Contributions
P.B. conceived the study. M.G., T.S. and P.B. designed experiments. M.G., F.C., T.S., A.G., C.P., E.M., D.D., R.B., S.A., S.H-A., S.J.L. and P.B. performed experiments. M.G., F.C., T.S., A.G., A.S., N.G. and P.B. interpreted the data. S.J.L. helped to identify the location of the pancreatic nerve in mice. J.-L.D. provided one of the external stimulators used in this study. P.B. wrote the manuscript and N.G. edited the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
A patent application is pending.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Material
Supplementary Figs. 1–14.
Rights and permissions
About this article
Cite this article
Guyot, M., Simon, T., Ceppo, F. et al. Pancreatic nerve electrostimulation inhibits recent-onset autoimmune diabetes. Nat Biotechnol 37, 1446–1451 (2019). https://doi.org/10.1038/s41587-019-0295-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41587-019-0295-8
This article is cited by
-
Neuromorphic electro-stimulation based on atomically thin semiconductor for damage-free inflammation inhibition
Nature Communications (2024)
-
Non-invasive imaging of sympathetic innervation of the pancreas in individuals with type 2 diabetes
Diabetologia (2024)
-
Pancreatic draining lymph nodes (PLNs) serve as a pathogenic hub contributing to the development of type 1 diabetes
Cell & Bioscience (2023)
-
Optogenetic stimulation of vagal nerves for enhanced glucose-stimulated insulin secretion and β cell proliferation
Nature Biomedical Engineering (2023)
-
Unravelling innervation of pancreatic islets
Diabetologia (2022)