Abstract
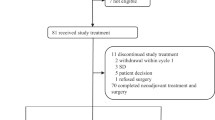
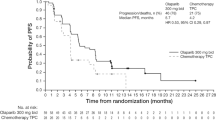
PARTNER is a prospective, phase II-III, randomised controlled clinical trial, which recruited patients with Triple Negative Breast Cancer (TNBC)1,2, who were gBRCA wild type (gBRCAwt)3. Patients (n=559) were randomised on a 1:1 basis to neoadjuvant carboplatin with paclitaxel +/- olaparib 150mg twice daily, days 3 to 14, for 4 cycles (gap schedule olaparib, research arm) followed by 3 cycles of anthracycline chemotherapy before surgery. The primary endpoint was pathological complete response (pCR)4, and secondary endpoints included event-free survival (EFS), and overall survival (OS)5. pCR was achieved in 51% in the research arm and 52% in the control arm (p=0.753). Estimated EFS at 36 months in research and control arms were 80% and 79% (log-rank p>0.9); OS were 90% and 87.2% (log-rank p=0.8) respectively. In patients with pCR, estimated EFS at 36 months was 90%, and with non-pCR was 70% (log-rank p < 0.001) and OS was 96% and 83% (log-rank p < 0.001) respectively. Neo-adjuvant olaparib did not improve pCR rates, EFS or OS when added to carboplatin/paclitaxel and anthracycline chemotherapy in patients with TNBC (gBRCAwt). This is in marked contrast to the major benefit of olaparib (gap schedule) in those with gBRCA pathogenic variants (gBRCAm) which is reported separately (gBRCAm article). ClinicalTrials.gov ID NCT03150576
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Rent or buy this article
Prices vary by article type
from$1.95
to$39.95
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Supplementary Information
This file contains: 1. Summary from protocol; and 2. PARTNER Trial Consortium Members.
Rights and permissions
About this article
Cite this article
Abraham, J.E., Pinilla, K., Dayimu, A. et al. The PARTNER trial of neoadjuvant olaparib in triple-negative breast cancer. Nature (2024). https://doi.org/10.1038/s41586-024-07384-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41586-024-07384-2
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.