Abstract
Baleen whales (mysticetes) use vocalizations to mediate their complex social and reproductive behaviours in vast, opaque marine environments1. Adapting to an obligate aquatic lifestyle demanded fundamental physiological changes to efficiently produce sound, including laryngeal specializations2,3,4. Whereas toothed whales (odontocetes) evolved a nasal vocal organ5, mysticetes have been thought to use the larynx for sound production1,6,7,8. However, there has been no direct demonstration that the mysticete larynx can phonate, or if it does, how it produces the great diversity of mysticete sounds9. Here we combine experiments on the excised larynx of three mysticete species with detailed anatomy and computational models to show that mysticetes evolved unique laryngeal structures for sound production. These structures allow some of the largest animals that ever lived to efficiently produce frequency-modulated, low-frequency calls. Furthermore, we show that this phonation mechanism is likely to be ancestral to all mysticetes and shares its fundamental physical basis with most terrestrial mammals, including humans10, birds11, and their closest relatives, odontocetes5. However, these laryngeal structures set insurmountable physiological limits to the frequency range and depth of their vocalizations, preventing them from escaping anthropogenic vessel noise12,13 and communicating at great depths14, thereby greatly reducing their active communication range.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Source data for figures are available at Zenodo (https://doi.org/10.5281/zenodo.10390075). The B. musculus recording is from Discovery of Sounds in the Sea, https://dosits.org/galleries/audio-gallery/marine-mammals/baleen-whales/blue-whale/. The B. mysticus recording is from the Watkins Marine Mammal Sound Database, Woods Hole Oceanographic Institution and the New Bedford Whaling Museum (https://whoicf2.whoi.edu/science/B/whalesounds/index.cfm). Source data are provided with this paper.
Code availability
Code is available at Zenodo (https://doi.org/10.5281/zenodo.10390075).
References
Clark, C. W. & Garland, E. C. (eds) Ethology and Behavioral Ecology of Mysticetes (Springer, 2022).
Reidenberg, J. S. & Laitman, J. T. Discovery of a low frequency sound source in Mysticeti (baleen whales): anatomical establishment of a vocal fold homolog. Anat. Rec. 290, 745–759 (2007).
Schoenfuss, H. L. et al. The anatomy of the larynx of the bowhead whale, Balaena mysticetus, and its sound-producing functions. Anat. Rec. 297, 1316–1330 (2014).
Damien, J. et al. Anatomy and functional morphology of the mysticete rorqual whale larynx: phonation positions of the U-fold. Anat. Rec. 302, 703–717 (2019).
Madsen, P. T., Siebert, U. & Elemans, C. P. H. Toothed whales use distinct vocal registers for echolocation and communication. Science 379, 928–933 (2023).
Reidenberg, J. S. in Ethology and Behavioral Ecology of Mysticetes (eds Clark, C. W. & Garland, E. C.) Ch. 3, 45–69 (Springer, 2022).
Adam, O. et al. New acoustic model for humpback whale sound production. Appl. Acoust. 74, 1182–1190 (2013).
Cazau, D., Adam, O., Aubin, T., Laitman, J. T. & Reidenberg, J. S. A study of vocal nonlinearities in humpback whale songs: from production mechanisms to acoustic analysis. Sci. Rep. 6, 31660 (2016).
Erbe, C. et al. Review of underwater and in-air sounds emitted by Australian and Antarctic marine mammals. Acoust. Aust. 45, 179–241 (2017).
Herbst, C. T., Elemans, C. P. H., Tokuda, I. T., Chatziioannou, V. & Švec, J. G. Dynamic system coupling in voice production. J. Voice https://doi.org/10.1016/j.jvoice.2022.10.004 (2023).
Elemans, C. P. H. et al. Universal mechanisms of sound production and control in birds and mammals. Nat. Commun. 6, 8978 (2015).
Findlay, C. R., Rojano-Doñate, L., Tougaard, J., Johnson, M. P. & Madsen, M. P. Small reductions in cargo vessel speed substantially reduce noise impacts to marine mammals. Sci. Adv. 9, eadf2987 (2023).
Erbe, C., Reichmuth, C., Cunningham, K., Lucke, K. & Dooling, R. Communication masking in marine mammals: a review and research strategy. Mar. Pollut. Bull. 103, 15–38 (2016).
Payne, R. & Webb, D. Orientation by means of long range acoustic signaling in baleen whales. Ann. NY Acad. Sci. 188, 110–141 (1971).
Huggenberger, S., Rauschmann, M. A. & Oelschlager, H. H. Functional morphology of the hyolaryngeal complex of the harbor porpoise (Phocoena phocoena): implications for its role in sound production and respiration. Anat. Rec. 291, 1262–1270 (2008).
Gil, K. N., Lillie, M. A., Vogl, A. W. & Shadwick, R. E. Rorqual whale nasal plugs: protecting the respiratory tract against water entry and barotrauma. J. Exp. Biol. 223, jeb219691 (2020).
Gil, K. N., Vogl, A. W. & Shadwick, R. E. Anatomical mechanism for protecting the airway in the largest animals on Earth. Curr. Biol. 32, 898–903 (2022).
Zhang, Z. Mechanics of human voice production and control. J. Acoust. Soc. Am. 140, 2614–2635 (2016).
Zhang, Z. Cause-effect relationship between vocal fold physiology and voice production in a three-dimensional phonation model. J. Acoust. Soc. Am. 139, 1493–1507 (2016).
Jiang, W. et al. High-fidelity continuum modeling predicts avian voiced sound production. Proc. Natl Acad. Sci. USA 117, 4718–4723 (2020).
Chhetri, D. K., Zhang, Z. & Neubauer, J. Measurement of Young’s modulus of vocal folds by indentation. J. Voice 25, 1–7 (2011).
Van den berg, J. Myoelastic-aerodynamic theory of voice production. J. Speech Hear. Res. 1, 227–244 (1958).
Baumgartner, M. F. et al. Low frequency vocalizations attributed to sei whales (Balaenoptera borealis). J. Acoust. Soc. Am. 124, 1339–1349 (2008).
Calderan, S. et al. Low-frequency vocalizations of sei whales (Balaenoptera borealis) in the Southern Ocean. J. Acoust. Soc. Am. 136, EL418–EL423 (2014).
Newhall, A. E., Lin, Y.-T., Lynch, J. F., Baumgartner, M. F. & Gawarkiewicz, G. G. Long distance passive localization of vocalizing sei whales using an acoustic normal mode approach. J. Acoust. Soc. Am. 131, 1814–1825 (2012).
McDonald, M. A. et al. Sei whale sounds recorded in the Antarctic. J. Acoust. Soc. Am. 118, 3941–3945 (2005).
Romagosa, M., Boisseau, O., Cucknell, A.-C., Moscrop, A. & McLanaghan, R. Source level estimates for sei whale (Balaenoptera borealis) vocalizations off the Azores. J. Acoust. Soc. Am. 138, 2367–2372 (2015).
Cazau, D., Adam, O., Laitman, J. T. & Reidenberg, J. S. Understanding the intentional acoustic behavior of humpback whales: a production-based approach. J. Acoust. Soc. Am. 134, 2268–2273 (2013).
Fitch, W. T., Neubauer, J. & Herzel, H. Calls out of chaos: the adaptive significance of nonlinear phenomena in mammalian vocal production. Anim. Behav. 63, 407–418 (2002).
Suthers, R. A., Fitch, W. T., Fay, R. R. & Popper, A. N. (eds) Vertebrate Sound Production and Acoustic Communication (Springer, 2016).
Reeb, D. & Best, P. B. Anatomy of the laryngeal apparatus of the pygmy right whale, Caperea marginata (Gray 1846). J. Morphol. 242, 67–81 (1999).
Titze, I., Riede, T. & Mau, T. Predicting achievable fundamental frequency ranges in vocalization across species. PLoS Comput. Biol. 12, e1004907 (2016).
Stimpert, A. K., Peavey, L. E., Friedlaender, A. S. & Nowacek, D. P. Humpback whale song and foraging behavior on an Antarctic feeding ground. PLoS ONE 7, e51214 (2012).
Dunlop, R. A., Noad, M. J., Cato, D. H. & Stokes, D. The social vocalization repertoire of east Australian migrating humpback whales (Megaptera novaeangliae). J. Acoust. Soc. Am. 122, 2893–2905 (2007).
Mercado, E. III, Schneider, J. N., Pack, A. A. & Herman, L. M. Sound production by singing humpback whales. J. Acoust. Soc. Am. 127, 2678–2691 (2010).
Tervo, O. M. et al. High source levels and small active space of high-pitched song in bowhead whales (Balaena mysticetus). PLoS ONE 7, e52072 (2012).
Širović, A., Hildebrand, J. A. & Wiggins, S. M. Blue and fin whale call source levels and propagation range in the Southern Ocean. J. Acoust. Soc. Am. 122, 1208–1215 (2007).
Orlikoff, R. F., Baken, R. J. & Kraus, D. H. Acoustic and physiologic characteristics of inspiratory phonation. J. Acoust. Soc. Am. 102, 1838–1845 (1997).
Anikin, A. & Reby, D. Ingressive phonation conveys arousal in human nonverbal vocalizations. Bioacoustics 31, 680–695 (2022).
McGowen, M. R. et al. Phylogenomic resolution of the cetacean tree of life using target sequence capture. Syst. Biol. 69, 479–501 (2020).
Garcia, M. & Herbst, C. T. Excised larynx experimentation: history, current developments, and prospects for bioacoustic research. Anthropol. Sci. 126, 9–17 (2018).
Pawlak, J. J. & Keller, D. S. Measurement of the local compressive characteristics of polymeric film and web structures using micro-indentation. Polym. Test. 22, 515–528 (2003).
Kumar, S., Liu, G., Schloerb, D. W. & Srinivasan, M. A. Viscoelastic characterization of the primate finger pad in vivo by microstep indentation and three-dimensional finite element models for tactile sensation studies. J. Biomech. Eng. 137, 061002 (2015).
Kelleher, J. E., Siegmund, T., Du, M., Naseri, E. & Chan, R. W. Empirical measurements of biomechanical anisotropy of the human vocal fold lamina propria. Biomech. Model. Mechanobiol. 12, 555–567 (2013).
Chan, R. W. & Titze, I. R. Effect of postmortem changes and freezing on the viscoelastic properties of vocal fold tissues. Ann. Biomed. Eng. 31, 482–491 (2003).
Sims, A. M. et al. Elastic and viscoelastic properties of porcine subdermal fat using MRI and inverse FEA. Biomech. Model. Mechanobiol. 9, 703–711 (2010).
Dhondt, G. The Finite Element Method for Three-Dimensional Thermomechanical Applications (Wiley, 2004).
Hunter, E. J. & Titze, I. R. Refinements in modeling the passive properties of laryngeal soft tissue. J. Appl. Physiol. 103, 206–219 (2007).
Arkowitz, R. & Rommel, S. Force and bending moment of the caudal muscles in the shortfin pilot whale. Mar. Mamm. Sci. 1, 203–209 (1985).
Peri, E., Falkingham, P. L., Collareta, A. & Bianucci, G. Biting in the Miocene seas: estimation of the bite force of the macroraptorial sperm whale Zygophyseter varolai using finite element analysis. Hist. Biol. 34, 1916–1927 (2022).
Smith, S. L. & Hunter, E. J. A viscoelastic laryngeal muscle model with active components. J. Acoust. Soc. Am. 135, 2041–2051 (2014).
Aroyan, J. L. et al. in Hearing by Whales and Dolphins (eds Au, W. W. L. et al.) Ch. 10, 409–469 (Springer, 2000).
Geng, B., Pham, N., Xue, Q. & Zheng, X. A three-dimensional vocal fold posturing model based on muscle mechanics and magnetic resonance imaging of a canine larynx. J. Acoust. Soc. Am. 147, 2597–2608 (2020).
Zollinger, S. A., Podos, J., Nemeth, E., Goller, F. & Brumm, H. On the relationship between, and measurement of, amplitude and frequency in birdsong. Anim. Behav. 84, e1–e9 (2012).
Dawbin, W. H. & Cato, D. H. Sounds of a pygmy right whale (Caperea marginata). Mar. Mamm. Sci. 8, 213–219 (1992).
Fahlman, A. et al. Comparative respiratory physiology in cetaceans. Front. Physiol. 11, 142 (2020).
Leith, D. E. Mammalian tracheal dimensions: scaling and physiology. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 55, 196–200 (1983).
Risch, D. et al. Mysterious bio-duck sound attributed to the Antarctic minke whale (Balaenoptera bonaerensis). Biol. Lett. 10, 20140175 (2014).
Gedamke, J., Costa, D. P. & Dunstan, A. Localization and visual verification of a complex minke whale vocalization. J. Acoust. Soc. Am. 109, 3038–3047 (2001).
Hosokawa, H. On the cetacean larynx, with special remarks on the laryngeal sack of the sei whale and the aryteno-epiglottideal tube of the sperm whale. Sci. Rep. Whales Res. Inst. 3, 23–62 (1950).
Viloria-Gómora, L., Romero-Vivas, E. & Urbán, J. R. Calls of Bryde’s whale (Balaenoptera edeni) recorded in the Gulf of California. J. Acoust. Soc. Am. 138, 2722–2725 (2015).
Širović, A., Bassett, H. R., Johnson, S. C., Wiggins, S. M. & Hildebrand, J. A. Bryde’s whale calls recorded in the Gulf of Mexico. Mar. Mamm. Sci. 30, 399–409 (2014).
Oleson, E. M., Barlow, J., Gordon, J., Rankin, S. & Hildebrand, J. A. Low frequency calls of Bryde’s whales. Mar. Mamm. Sci. 19, 407–419 (2003).
Wang, Z.-T. et al. Vocalization of Bryde’s whales (Balaenoptera edeni) in the Beibu Gulf, China. Mar. Mamm.Sci. 38, 1118–1139 (2022).
Edds, P. L., Odell, D. K. & Tershy, B. R. Vocalizations of a captive juvenile and free‐ranging adult‐calf pairs of Bryde’s whales, Balaenoptera edeni. Mar. Mamm. Sci. 9, 269–284 (1993).
Calambokidis, J. et al. Insights into the underwater diving, feeding, and calling behavior of blue whales from a suction-cup-attached video-imaging tag (CRITTERCAM). Mar. Technol. Soc. J. 41, 19–29 (2007).
Mellinger, D. K. & Clark, C. W. Blue whale (Balaenoptera musculus) sounds from the North Atlantic. J. Acoust. Soc. Am. 114, 1108–1119 (2003).
Lewis, L. A. et al. Context-dependent variability in blue whale acoustic behaviour. R. Soc. Open Sci. 5, 180241 (2018).
Oleson, E. M. et al. Behavioral context of call production by eastern North Pacific blue whales. Mar. Ecol. Prog. Ser. 330, 269–284 (2007).
Watkins, W. A. Activities and underwater sounds of fin whales. Sci. Rep. Whales Res. Inst. 33, 83–117 (1981).
Stimpert, A. K. et al. Sound production and associated behavior of tagged fin whales (Balaenoptera physalus) in the Southern California Bight. Anim. Biotelemetry 3, 23 (2015).
Weirathmueller, M. J., Wilcock, W. S. D. & Soule, D. C. Source levels of fin whale 20 Hz pulses measured in the Northeast Pacific Ocean. J. Acoust. Soc. Am. 133, 741–749 (2013).
Mercado, E. III & Perazio, C. E. All units are equal in humpback whale songs, but some are more equal than others. Anim. Cogn. 25, 149–177 (2022).
Videsen, S. K. A., Bejder, L., Johnson, M., Madsen, P. T. & Goldbogen, J. High suckling rates and acoustic crypsis of humpback whale neonates maximise potential for mother–calf energy transfer. Funct. Ecol. 31, 1561–1573 (2017).
Burnham, R., Duffus, D. & Mouy, X. Gray whale (Eschrictius robustus) call types recorded during migration off the west coast of Vancouver Island. Front. Mar. Sci. 5, 00329 (2018).
López-Urbán, A., Thode, A., Durán, C. B., Urbán R, J. & Swartz, S. Two new grey whale call types detected on bioacoustic tags. J. Mar. Biol. Assoc. UK 98, 1169–1175 (2018).
Rankin, S. & Barlow, J. Source of the North Pacific “boing” sound attributed to minke whales. J. Acoust. Soc. Am. 118, 3346–3351 (2005).
Schevill, W. E. & Watkins, W. A. Intense low-frequency sounds from an Antarctic minke whale, Balaenoptera acutorostrata. Breviora 388, 1–8 (1972).
Edds-Walton, P. L. Vocalizations of minke whales Balaenoptera acutorostrata in the St. Lawrence estuary. Bioacoustics 11, 31–50 (2000).
Dominello, T. & Širović, A. Seasonality of Antarctic minke whale (Balaenoptera bonaerensis) calls off the western Antarctic Peninsula. Mar. Mamm. Sci. 32, 826–838 (2016).
Širović, A. et al. North Pacific right whales (Eubalaena japonica) recorded in the northeastern Pacific Ocean in 2013. Mar. Mamm. Sci. 31, 800–807 (2015).
Mellinger, D. K., Stafford, K. M., Moore, S. E., Munger, L. & Fox, C. G. Detection of North Pacific right whale (Eubalaena japonica) calls in the Gulf of Alaska. Mar. Mamm. Sci. 20, 872–879 (2004).
Crance, J. L., Berchok, C. L., Wright, D. L., Brewer, A. M. & Woodrich, D. F. Song production by the North Pacific right whale, Eubalaena japonica. J. Acoust. Soc. Am. 145, 3467–3479 (2019).
Trygonis, V., Gerstein, E., Moir, J. & McCulloch, S. Vocalization characteristics of North Atlantic right whale surface active groups in the calving habitat, southeastern United States. J. Acoust. Soc. Am. 134, 4518–4531 (2013).
Dombroski, J. R. G., Parks, S. E., Groch, K. R., Flores, P. A. C. & Sousa‐lima, R. S. Upcall production by southern right whale (Eubalaena australis) mother‐calf pairs may be independent of diel period in a nursery area. Mar. Mamm. Sci. 33, 669–677 (2017).
Dombroski, J. R., Parks, S. E., Groch, K. R., Flores, P. A. & Sousa-Lima, R. S. Vocalizations produced by southern right whale (Eubalaena australis) mother-calf pairs in a calving ground off Brazil. J. Acoust. Soc. Am. 140, 1850–1857 (2016).
Clark, C. W. The acoustic repertoire of the southern right whale, a quantitative analysis. Anim. Behav. 30, 1060–1071 (1982).
Erbs, F., van der Schaar, M., Weissenberger, J., Zaugg, S. & André, M. Contribution to unravel variability in bowhead whale songs and better understand its ecological significance. Sci. Rep. 11, 168 (2021).
Thode, A. M. et al. Source level and calling depth distributions of migrating bowhead whale calls in the shallow Beaufort Sea. J. Acoust. Soc. Am. 140, 4288–4297 (2016).
Acknowledgements
We thank staff at the Natural History Museum of Denmark, the Fisheries and Maritime Museum and the Danish Nature Agency for their aid in stranding response and dissection of the sei and humpback whales; C. Bie Thøstesen and M. Tange Olsen for help in organizing the dissections; G. Hantke and A. Kitchener for providing the minke whale larynx; C. Herbst and D. Mann for assistance with experiments in Vienna; and L. Jakobsen, P. T. Madsen and D. Wisniewska for comments on the manuscript. Funding was from Carlsberg Foundation CF14-1096 and NovoNordisk grant NFF20OC0063964 to C.P.H.E. and an Austrian Science Fund (FWF) grant W1262-B29 to W.T.F.
Author information
Authors and Affiliations
Contributions
C.P.H.E., M.H.J. and M.W. carried out experiments on excised larynx in Odense, and C.P.H.E., H.P. and W.T.F. in Vienna. C.P.H.E., B.R.M. and J.N. scanned the preparations and M.H.J. and H.P. annotated the scans. C.P.H.E. designed and built the experimental set-ups in Odense, analysed the experimental data and made figures. W.J., X.Z., C.P.H.E. and Q.X. developed the FSI model. W.J. analysed simulation data and made figures. C.P.H.E. carried out and analysed the material tests. C.P.H.E. and W.T.F. wrote the first drafts of the manuscript, and all authors contributed to the final draft.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Nicholas Pyenson, Joy Reidenberg and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
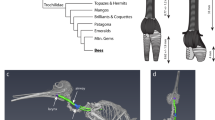
Extended Data Fig. 1 Sei whale larynx gross anatomy.
a, 3D render of laryngeal cartilages based on CT scan as in Fig. 1d. b, Ventral view of larynx defrosted after in vitro phonation experiment. Laryngeal sac has been cut medially and the blue tracheal connector is visible. c, Dorsal view of larynx with cricoid cartilage cut and arytenoids bend laterally. d, View on Cricoid Cushion (CC) and Transverse arytenoid folds (TAF) in rest (left) and adducted against each other as during phonation (right). e, Sagittal sections through the CC at various adjacent locations showing the tensor pulvini muscle (deep red) and CC fat (yellow to greenish). Section 7 and 8 have already been frozen in liquid nitrogen. f, 3D render and g, dissection view of medial section through larynx. Indicated are CC sections and TAF sections through the arytenoid cartilage (below).
Extended Data Fig. 2 Humpback whales phonate by CC against TAF vibration.
a, Still of endoscopic view. b, Overview and c, detail of small section of the in vitro sound production data showing that the CC and TAFs vibrate and that sound and acceleration excitation occurs on gap opening.
Extended Data Fig. 3 Tissue motion during phonation on CC and TAF mucosa.
Motion during phonation at Probe 1 and 2 location (Fig. 3f) is larger on CC compared to TAF mucosa (1.84 ± 0.08 vs. 0.32 ± 0.01 mm in Probe 1 location, n = 11 cycles; two-tailed, paired t-test, p = 6.2e−10).
Extended Data Fig. 4 Ingressive flow does not lead to stable self-sustained oscillations of CC against TAF.
a, Laryngeal flow waveform and b, probe readout showing a damped vibration, instead of self-sustained oscillations. See also Supplementary Video 6.
Extended Data Fig. 5 Stimulation of vocalis muscle does not have a notable effect on vocalization frequency.
Three different stimulation levels (α) of the vocalis muscle: a, α = 0; b, α = 0.1; c, α = 0.2. Left, the muscle stress in the fibre direction; Right: Laryngeal flow as a function of time. The fundamental frequency (fo) was obtained by a fast fourier transform of the data from 50 ms to 400 ms.
Extended Data Fig. 6 Strain-stress relationship of the passive component of the modelled muscle tissue.
Left, transverse direction. Right, along the fibre direction.
Supplementary information
Supplementary Audio 1
Aerial sound signal of CC against TAF phonation in sei whale.
Supplementary Audio 2
Acceleration signal of CC against TAF phonation in sei whale.
Supplementary Audio 3
Electroglottograph signal of CC against TAF phonation in sei whale.
Supplementary Audio 4
Aerial sound signal of CC against TAF phonation in minke whale.
Supplementary Audio 5
Electroglottograph signal of CC against TAF phonation in minke whale.
Supplementary Audio 6
Aerial sound signal of CC against TAF phonation in humpback whale.
Supplementary Audio 7
Acceleration signal of CC against TAF phonation in humpback whale.
Supplementary Audio 8
Electroglottograph signal of CC against TAF phonation in humpback whale.
Supplementary Audio 9
Aerial sound signal of bilateral TAF phonation in humpback whale.
Supplementary Audio 10
Acceleration signal of bilateral TAF phonation in humpback whale.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elemans, C.P.H., Jiang, W., Jensen, M.H. et al. Evolutionary novelties underlie sound production in baleen whales. Nature 627, 123–129 (2024). https://doi.org/10.1038/s41586-024-07080-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-024-07080-1
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.