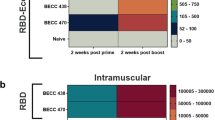
Abstract
The COVID-19 pandemic has fostered major advances in vaccination technologies1,2,3,4; however, there are urgent needs for vaccines that induce mucosal immune responses and for single-dose, non-invasive administration4,5,6. Here we develop an inhalable, single-dose, dry powder aerosol SARS-CoV-2 vaccine that induces potent systemic and mucosal immune responses. The vaccine encapsulates assembled nanoparticles comprising proteinaceous cholera toxin B subunits displaying the SARS-CoV-2 RBD antigen within microcapsules of optimal aerodynamic size, and this unique nano–micro coupled structure supports efficient alveoli delivery, sustained antigen release and antigen-presenting cell uptake, which are favourable features for the induction of immune responses. Moreover, this vaccine induces strong production of IgG and IgA, as well as a local T cell response, collectively conferring effective protection against SARS-CoV-2 in mice, hamsters and nonhuman primates. Finally, we also demonstrate a mosaic iteration of the vaccine that co-displays ancestral and Omicron antigens, extending the breadth of antibody response against co-circulating strains and transmission of the Omicron variant. These findings support the use of this inhaled vaccine as a promising multivalent platform for fighting COVID-19 and other respiratory infectious diseases.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The sequence data reported in this study have been archived in the Sequence Read Archive under accession numbers PRJNA813749 and PRJNA1002859. All other data supporting the findings of this study are available in the Article and its Supplementary Information and are also available from the corresponding author on request. Source data are provided with this paper.
References
Lambert, H. et al. COVID-19 as a global challenge: towards an inclusive and sustainable future. Lancet Planet Health 4, E312–E314 (2020).
Yang, J. et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature 586, 572–577 (2020).
Zhang, N. N. et al. A thermostable mRNA vaccine against COVID-19. Cell 182, 1271–1283.e1216 (2020).
Hassan, A. O. et al. A single-dose intranasal ChAd vaccine protects upper and lower respiratory tracts against SARS-CoV-2. Cell 183, 169–184.e113 (2020).
Amorij, J. P. et al. Pulmonary delivery of an inulin-stabilized influenza subunit vaccine prepared by spray-freeze drying induces systemic, mucosal humoral as well as cell-mediated immune responses in BALB/c mice. Vaccine 25, 8707–8717 (2007).
Waltz, E. How nasal-spray vaccines could change the pandemic. Nature 609, 240–242 (2022).
Petersen, E. et al. Comparing SARS-CoV-2 with SARS-CoV and influenza pandemics. Lancet Infect. Dis. 20, e238–e244 (2020).
Wu, F. et al. A new coronavirus associated with human respiratory disease in China. Nature 579, 265–269 (2020).
Gao, Q. et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 369, 77–81 (2020).
Wang, H. et al. Development of an inactivated vaccine candidate, BBIBP-CorV, with potent protection against SARS-CoV-2. Cell 182, 713–721.e719 (2020).
Dai, L. et al. A universal design of betacoronavirus vaccines against COVID-19, MERS, and SARS. Cell 182, 722–733.e711 (2020).
Corbett, K. S. et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N. Engl. J. Med. 383, 1544–1555 (2020).
Folegatti, P. M. et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 396, 467–478 (2020).
Chen, J. et al. A live attenuated virus-based intranasal COVID-19 vaccine provides rapid, prolonged, and broad protection against SARS-CoV-2. Sci. Bull. 67, 1372–1387 (2022).
Bricker, T. L. et al. A single intranasal or intramuscular immunization with chimpanzee adenovirus-vectored SARS-CoV-2 vaccine protects against pneumonia in hamsters. Cell Rep. 36, 109400 (2021).
Le Nouen, C. et al. Intranasal pediatric parainfluenza virus-vectored SARS-CoV-2 vaccine is protective in monkeys. Cell 185, 4811–4825.e4817 (2022).
Ponce-de-Leon, S. et al. Safety and immunogenicity of a live recombinant Newcastle disease virus-based COVID-19 vaccine (Patria) administered via the intramuscular or intranasal route: Interim results of a non-randomized open label phase I trial in Mexico. Preprint at medRxiv 10.1101/2022.02.08.22270676 (2022).
Afkhami, S. et al. Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. Cell 185, 896–915.e819 (2022).
Wu, S. et al. Safety, tolerability, and immunogenicity of an aerosolised adenovirus type-5 vector-based COVID-19 vaccine (Ad5-nCoV) in adults: preliminary report of an open-label and randomised phase 1 clinical trial. Lancet Infect. Dis. 21, 1654–1664 (2021).
Jeyanathan, M. et al. Aerosol delivery, but not intramuscular injection, of adenovirus-vectored tuberculosis vaccine induces respiratory-mucosal immunity in humans. JCI Insight 7, e155655 (2022).
Kunda, N. K., Somavarapu, S., Gordon, S. B., Hutcheon, G. A. & Saleem, I. Y. Nanocarriers targeting dendritic cells for pulmonary vaccine delivery. Pharm. Res. 30, 325–341 (2013).
Patton, J. S. & Byron, P. R. Inhaling medicines: delivering drugs to the body through the lungs. Nat. Rev. Drug Discov. 6, 67–74 (2007).
Smits, H. H. et al. Cholera toxin B suppresses allergic inflammation through induction of secretory IgA. Mucosal Immunol. 2, 331–339 (2009).
Wakabayashi, A., Shimizu, M., Shinya, E. & Takahashi, H. HMGB1 released from intestinal epithelia damaged by cholera toxin adjuvant contributes to activation of mucosal dendritic cells and induction of intestinal cytotoxic T lymphocytes and IgA. Cell Death Dis. 9, 631 (2018).
Pan, C. et al. Biosynthesis of self-assembled proteinaceous nanoparticles for vaccination. Adv. Mater. 32, e2002940 (2020).
Reddy, S. T. et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 25, 1159–1164 (2007).
Li, X. et al. Orthogonal modular biosynthesis of nanoscale conjugate vaccines for vaccination against infection. Nano Res. 15, 1645–1653 (2022).
Heida, R., Hinrichs, W. L. & Frijlink, H. W., Inhaled vaccine delivery in the combat against respiratory viruses: a 2021 overview of recent developments and implications for COVID-19. Expert Rev. Vaccines 21, 957–974 (2022).
Pulliam, B., Sung, J. C. & Edwards, D. A. Design of nanoparticle-based dry powder pulmonary vaccines. Expert Opin. Drug Deliv. 4, 651–663 (2007).
Xi, X. B. et al. Self-healing microcapsules synergetically modulate immunization microenvironments for potent cancer vaccination. Sci. Adv. 6, eaay7735 (2020).
Xie, X. L. et al. Therapeutic vaccination against leukaemia via the sustained release of co-encapsulated anti-PD-1 and a leukaemia-associated antigen. Nat. Biomed. Eng. 5, 414–428 (2021).
Liao, M. et al. Single-cell landscape of bronchoalveolar immune cells in patients with COVID-19. Nat. Med. 26, 842–844 (2020).
Toor, S. M., Saleh, R., Sasidharan Nair, V., Taha, R. Z. & Elkord, E. T-cell responses and therapies against SARS-CoV-2 infection. Immunology 162, 30–43 (2021).
Grifoni, A. et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell 181, 1489–1501.e1415 (2020).
Yu, D., Walker, L. S. K., Liu, Z., Linterman, M. A. & Li, Z. Targeting TFH cells in human diseases and vaccination: rationale and practice. Nat. Immunol. 23, 1157–1168 (2022).
Madhavan, M. et al. Tolerability and immunogenicity of an intranasally-administered adenovirus-vectored COVID-19 vaccine: An open-label partially-randomised ascending dose phase I trial. eBioMedicine 85, 104298 (2022).
Zhu, F. C. et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 396, 479–488 (2020).
Li, J. X. et al. Safety and immunogenicity of heterologous boost immunisation with an orally administered aerosolised Ad5-nCoV after two-dose priming with an inactivated SARS-CoV-2 vaccine in Chinese adults: a randomised, open-label, single-centre trial. Lancet Respir. Med. 10, 739–748 (2022).
Zhou, Q. T., Tang, P., Leung, S. S., Chan, J. G. & Chan, H. K. Emerging inhalation aerosol devices and strategies: where are we headed? Adv. Drug Deliv. Rev. 75, 3–17 (2014).
Walters, A. A., Krastev, C., Hill, A. V. S. & Milicic, A. Next generation vaccines: single-dose encapsulated vaccines for improved global immunisation coverage and efficacy. J. Pharm. Pharmacol. 67, 400–408 (2015).
Finak, G. et al. MAST: a flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. Genome Biol. 16, 278 (2015).
Li, Q. et al. The impact of mutations in SARS-CoV-2 spike on viral infectivity and antigenicity. Cell 182, 1284–1294.e1289 (2020).
Nie, J. et al. Quantification of SARS-CoV-2 neutralizing antibody by a pseudotyped virus-based assay. Nat. Protoc. 15, 3699–3715 (2020).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant nos. T2225021, 21821005, 32030062, U2001224, 81930122, U20A20361 and 82202028), Beijing Natural Science Foundation (JQ21027), CAS Project for Young Scientists in Basic Research (YSBR-083), National Key Research and Development Program of China (2021YFC2302600), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB29040303), CAMS Innovation Fund for Medical Science (2021-I2M-1-024), and the Major Science and Technology Special Projects of Yunnan Province (202002AA100009). We thank X. Zhao for providing wild-type and Omicron pseudotyped viruses and B. Wang for providing the SARS-CoV-2 RBD peptide pool.
Author information
Authors and Affiliations
Contributions
W.W., G.M., L.Z. and H.W. conceived, designed and supervised the experiments. T.Y., Z.J. and X.L. performed the experiments. Youchun Wang and W.H. provided most pseudotyped viruses and guided pseudovirus neutralization assays. Y.H., Y.Z. and E.W. contributed to live virus neutralization assays. Z.H., Y.L., F.Y. and X.Z. contributed to live virus challenge and transmission experiments. Y.F. provided the convalescent sera and provided professional advice on immunologic evaluation. M.Q. and D.Y. provided constructive suggestions and opinions on immunologic experiment design. W.Y. and L.H. contributed to evaluation of pulmonary delivery. Y.Q. guided immunologic evaluation on nonhuman primates. T.Y., Z.J., X.L., S.W., H.Y. and Yishu Wang, aided in data analysis. T.Y., Z.J., X.L. and W.W. wrote the manuscript. W.W., G.M., L.Z. and H.W. revised the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
H.W. and L.Z. are the inventors on patent application ZL202010175401.6 held or submitted by the Beijing Institute of Biotechnology related to CTB nanoparticles. G.M. is an inventor on patent application ZL201110401710.1 held or submitted by the Institute of Processing and Engineering related to porous microcapsules. The other authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Construction and antigenicity characterization of RBD-conjugated CNP.
(a) Western blotting analysis of covalent complex formation between SC-RBD and ST-CNP. SC-RBD, ST-CNP, and R-CNP were assessed using antibodies against CTB. (b) DLS analysis of CNP and R-CNP. (c) Comparison of hACE2 binding affinity among RBD, SC-RBD, and R-CNP. Affinity was assessed using a competition ELISA. (d) Ka, Kd, and KD of unconjugated RBD and R-CNP determined by surface plasmon resonance assays. Ka: Association rate. Kd: Dissociation rate. KD: Binding affinity constant calculated as Kd/Ka. (e) Flow cytometry analysis of known surface markers to assess the activation of bone marrow dendritic cells (BMDCs) after treatment with RBD, CTB + RBD, or R-CNPs. CTB + RBD: mixture of free CTB and RBD. (f) Flow cytometry analysis of known surface markers to assess the activation of macrophages after treatment with RBD, CTB + RBD, or R-CNP. (g) Heatmap of cytokine secretion by APCs after treatment with RBD, CTB + RBD, or R-CNP. Data in c (n = 3 biologically independent samples), e and f (n = 3 biologically independent cells) are shown as the mean ± SEM. Significance was calculated by one-way ANOVA with multiple comparison tests in e and f.
Extended Data Fig. 2 Optimization and characterization of the inhalable R-CNP@M vaccine.
(a) Schematic illustrations of inhaled microcapsule preparation, encapsulation, and characterization. Both the particle size and porosity were adjusted to obtain a suitable aerodynamic diameter to enable efficient alveoli delivery (left panel). The interior structure was optimized for good penetration of R-CNPs and complete sealing of open pores (middle panel). Then, the prepared R-CNP@M was characterized to evaluate the loading performance, morphological features, release profile, and remaining antigen bioactivity (right panel). (b) Fitting surface among aerodynamic size (da), hydrodynamic size (dh) and porosity (po.). The magenta area represents microcapsules with aerodynamic sizes ranging from 1–4 μm. (c) Evaluation of the microencapsulation process. Microspheres with similar hydrodynamic sizes and porosities (po.) but with various pore sizes (red) were prepared and mixed with antigen (blue) followed by self-healing. The different performances on penetration of R-CNPs and self-healing of microcapsules resulted in different encapsulation efficiencies. (d) In vitro release profile of R-CNPs from microcapsules. (e) Aerodynamic size of fresh R-CNP@M and lyophilized R-CNP@M stored for 1 month at room temperature (RT). (f) DLS analysis of the harvested R-CNPs from lyophilized R-CNP@M stored for 1 month at RT. Data in c and d (n = 4 biologically independent samples) are shown as the mean ± SEM.
Extended Data Fig. 3 Safety evaluations and in vivo characterizations of R-CNP@M in mice.
(a) Endotoxin levels of R-CNPs and R-CNPs@M. The dose of mice was 10 μg. (b) Respiratory function evaluation of vaccinated mice at the indicated time points. (c) Haemogram indices of vaccinated mice at the indicated time points. (d) H&E staining of lung tissues and other main organs at the indicated time points. All images are shown at the same magnification. (e) Changes of cytokine levels in BALF at the indicated time points. (f) Representative images of the Cy7-labelled R-CNP signal in the main organs at the indicated time points. (g) Calculation of the area under the curve (AUC) in Fig. 1j. (h) Quantitative distribution of R-CNP@M in various lung structures. (i) Distribution of R-CNP (labelled with Cy5) in healed microcapsules extracted from bronchoalveolar lavage fluid (BALF) at the indicated time points. Data in a, c, e (n = 3), g (n = 6) and h (n = 6, 39, and 46 among the trachea, bronchi, and alveoli, respectively) are shown as the mean ± SEM. The violin plot in b shows the sampling data of 6 mice over 10 min, with central values indicating the mean and whiskers display the interquartile range. Significance was calculated using one-way ANOVA with multiple comparison tests in e and h and two-sided unpaired t-test in g.
Extended Data Fig. 4 Analysis of immune cell status in the lungs of immunized mice.
(a) Bubble chart of canonical cell-type markers of nine major cell types. (b) Proportion of macrophage and DC of mice from the PBS, CNP, R-CNP, and R-CNP@M groups. (c) Heatmap of differentially expressed genes (DEGs) among the PBS, CNP, R-CNP, and R-CNP@M groups from macrophages and DCs in the lungs. (d and e) KEGG (Kyoto Encyclopedia of Genes and Genomes) terms related to the DCs (d) and macrophages (e) activation enriched by DEGs between R-CNP@M and PBS. (f) Annotated T-cell clusters are depicted using t-distributed stochastic neighbour embedding (t-SNE) plots for the merged scRNA-seq data (combined with the PBS, CNP, R-CNP, and R-CNP@M groups). The defined T-cell subsets are naïve CD4+ T, naïve CD8+ T, effector and/or memory CD4+ T (E&M CD4+ T), effector and/or memory CD8+ T (E&M CD8+ T), γδ T, NK T, and regulatory T (Treg) cells. (g) DEGs from E&M CD8+ T cells are represented by volcano plots for R-CNP@M vs. PBS. up: up regulation; down: down regulation; ns, no significance. (h) GO (Gene Ontology) terms from biological processes related to the activation of E&M CD8+ T cells enriched by DEGs between R-CNP@M and PBS. (i) Annotated B-cell clusters are depicted using t-SNE plots for the merged scRNA-seq data (combined with PBS, CNP, R-CNP, and R-CNP@M groups). The defined B-cell subsets are memory B, plasma, naïve B, germinal centre B and other B cells. (j) DEGs from memory B cells are represented by volcano plots for R-CNP@M vs. PBS. (k) GO terms from biological processes related to the activation of memory B cells enriched by DEGs between R-CNP@M and PBS. The upregulated and downregulated DEGs were obtained by Model-based Analysis of Single-cell Transcriptomics (MAST) analysis in g and j.
Extended Data Fig. 5 Additional evaluations of T-cell responses in the respiratory tract.
(a) Proportion of memory CD8+ T cells (CD44+ CD8+) in the lung (among CD8+ T cells, assessed by flow cytometry) on Day 70. (b) Proportion of tissue-resident memory T cells (TRM) (CD69+ CD103+) in the lung (among CD44+ CD8+ T cells, assessed by flow cytometry) on Day 70. (c) ELIspot assay for determination of the immunogenicity of overlapping peptides. SPC: spot-forming cells. (d) Immune Epitope Database analysis (IEDB) prediction scores of the predicted RBD-specific CD8+ T-cell epitopes. (e) IEDB prediction scores of the predicted RBD-specific CD4+ T-cell epitopes. (f) Verification of the immunogenicity of the candidate CD8+ and CD4+ T-cell epitopes (CGPKKSTNL and VGGNYNYLYRLFRKS for CD8+ and CD4+ T cells, respectively, and assessed by flow cytometry). (g) Proportion of IFN-γ+ TRM with/without RBD epitope stimulation in the lung (among CD69+ CD103+ T cells, assessed by flow cytometry) on Day 70. (h) Proportion of IFN-γ+ CD4+ TRM with/without RBD epitope stimulation in the lung (among CD69+ CD11a+ T cells, assessed by flow cytometry) on Days 21 (left) and 70 (right). (i) Cell numbers of CD4+ and CD8+ T cells in NALT. (j) Epitope-specific T-cell responses in NALT on Day 21. (k) Epitope-specific T-cell responses in NALT on Day 70. (l) Cell numbers of CD4+ and CD8+ T cells in nose. Data in a-c and f-l (n = 3 mice per group) are shown as the mean ± SEM. Significance was calculated using a one-way ANOVA with multiple comparison tests in a, b and two-sided unpaired t-test in g, h, j and k.
Extended Data Fig. 6 Additional evaluations of antibody responses in mice.
(a) Endpoint titers of serum IgG1 and IgG2a determined by ELISA on Days 14, 28, 42, 56, 70, 91 133, 175, 266, and 365. (b) Endpoint titers of serum IgG, IgG1 and IgG2a determined by ELISA on Days 14 and 70. (c) Measurement of pVNT50 from serum, with sampling on Days 14 and 70. (d) Serum antibody quality analysis of R-CNP@M (indicated by the ratio of pVNT50/serum IgG titer) on Days 28, 56, 70, 133, 266, and 365. The means of each time point were connected. (e) Relative counts for IGHC/V/D/J gene (frequency > 0.005) usage and pairing in pulmonary BCR repertoires in the R-CNP and R-CNP@M groups on Day 70. The R-CNP@M group shows more high-frequency IGH CDR3 pairing types and more IGHG and IGHA transcripts. (f) Venn diagram among the four indicated groups. There are 1238 convergent clonotypes between the 14 days post vaccination (dpv) and 70 dpv samples for the R-CNP group, 1041 convergent clonotypes between 14 dpv and 70 dpv samples for the R-CNP@M group, and 350 convergent clonotypes among the four samples. (g) Normalized frequency of R-CNP-specific clonotypes among the four samples. The convergent clonotypes among four samples were defined as R-CNP-specific clones. (h) Distribution of CDR3 heavy-chain variable (VH) gene germline divergence of R-CNP-specific clones. (i) Statistical analysis of somatic hypermutation (SHM) in the R-CNP-specific clones. (j) Genealogy tree of Top 5 convergent clone sequences. The Top 5 among R-CNP-specific clonotypes in each sample were selected and analyzed by MEGA 11.0 using Neighbor-joining statistical method. (k) Endpoint titers of nasal lavage fluid IgA measured by ELISA on Days 70. Data in a-d, k (n = 6) are shown as the mean ± SEM. Significance was calculated using two-sided Pearson chi-square test in i, using Kruskal-Wallis test with multiple comparison in k.
Extended Data Fig. 7 Immunologic performance evaluations of R-CNP@M against SARS-CoV-2 in mice.
(a) Schematic illustration of immunization, antibody intervention, and sampling in mice. (b) Proportion of CD8+ T cells in the lung (among total T cells, assessed by flow cytometry) on Day 28. (c) Proportion of GC B cells (GL7+ IgD− B220+) in mLNs (assessed by flow cytometry) on Day 28. (d) Proportion of CD8+ T cells in mLNs (assessed by flow cytometry) on Day 28. (e) Endpoint titers of serum IgG measured by ELISA on Day 28. (f) Measurement of serum pVNT50 against the D614G strain on Day 28. (g) Percentage of weight loss compared with the initial weight on Day 0 after the wild-type strain challenge. (h) Survival curve of mice post-SARS-CoV-2 challenge. (i) Viral RNA levels measured by qPCR (using ORF1a/b primers) in the lung and nose homogenate from surviving mice at 4 days post-challenge. Different numbers of mice died in the IsoAb, Bdep, and Tdep groups within 4 days post-challenge, and all remaining surviving mice were euthanized on the fourth day post-challenge. (j) Immunohistochemical analysis of the SARS-CoV-2 N protein in lung tissues sections. The arrows indicate the SARS-CoV-2 N protein-positive areas. (k) Histopathology analysis of lung tissues sections. Data in e-g (n = 8) and i (n = 4, 6, 7, and 8 among the IsoAb, Bdep, Tdep, and Naïve groups, respectively) are shown as the mean ± SEM. Significance was calculated using Kruskal-Wallis test with multiple comparison in e, f, g, and i.
Extended Data Fig. 8 Comparisons of Zifivax and R-CNP@M on immunologic performance against SARS-CoV-2 in hamsters.
The hamsters immunized with Zifivax (10 μg equivalent RBD, immunized three times with an interval of 2 weeks, licensed recombinant protein subunit vaccine) received the same equivalent RBD antigen as mice immunized with R-CNP@M (30 μg equivalent RBD, immunized once). (a) Schematic illustration of immunization and sampling in hamsters. (b) Endpoint titers of serum IgG determined by ELISA on Days 14, 28 and 42. (c) Measurement of MN50 from serum, with sampling on Day 42. (d) Measurement of MN50 from BALF, with sampling on Day 42. (e) Viral RNA levels determined in the lung and nose tissues by qPCR (using ORF1a/b primers). (f) Viral titers assessed in lung tissues at 3 dpi. (g) Immunohistochemical analysis and histopathology analysis of the SARS-CoV-2 N protein in lung tissues sections at 3 dpi. The arrows indicate the SARS-CoV-2 N protein-positive areas. Data in b-f (n = 6) are shown as the mean ± SEM. Significance was calculated using two-sided Mann-Whitney tests in c-f.
Extended Data Fig. 9 Additional evaluations of R-CNP@M in cynomolgus monkeys.
(a) Immunofluorescent imaging of germinal centers in mLNs. The nuclei (blue) and B cells (red) were labelled with DAPI and B220 antibodies, respectively. Proliferation was indicated by the expression of Ki-67 (green). (b, c) Biochemical (b) and physiological indices (c) of blood in cynomolgus monkeys. AST: aspartate aminotransferase; LDH: lactate dehydrogenase; ALP: alkaline phosphatase; TP: total protein; BUN: blood urea nitrogen. (d) Proportion of granzyme B+ CD8+ T cells (Gzmb+ CD8+) among CD8+ T cells in peripheral blood. (e) Table showing binding antibody titers (in WHO BAU/mL) from serum in nonhuman primates on Day 56. We included the WHO International standard for anti-SARS-CoV-2 immunoglobulin (NIBSC code: 21/340) to calibrate binding antibody titers. Data in b-d (n = 5) are shown as the mean ± SEM. Significance was calculated using two-sided unpaired t tests in b-d.
Extended Data Fig. 10 Additional evaluations of mosaic vaccine.
(a) Endpoint titers of BALF IgG from mice vaccinated with the mosaic vaccine in Fig. 6 determined by ELISA on Day 28. (b) Correlations of IgG antibody titers and pVNT50 in serum. (c) Correlations of IgA antibody titers and pVNT50 in BALF. (d) Correlations of IgG antibody titer and pVNT50 in BALF. (e) Endpoint titers of IgG on Day 14 after booster immunization with mosaic vaccine (RWTRO-CNP@M) of mice that were prime-immunized with RWT-CNP@M. (f) Endpoint titers of IgG on Day 14 after booster immunization with mosaic vaccine (RWTRO-CNP@M) of cynomolgus monkeys that were prime-immunized with RWT-CNP@M. (g) Endpoint titers of serum IgG determined by ELISA on Day 42. (h) Measurement of pVNT50 from serum, with sampling on Day 42. (i) Measurement of pVNT50 from BALF, with sampling on Day 42. (j) Schematic illustration of the contact/airborne transmission protection model. (k) Viral RNA levels measured in lung homogenates by qPCR (using ORF1a/b primers). (l) Viral titers assessed in lung homogenates at 3 days post-exposure (dpe). (m) Histopathology analysis and pathological scores of lung tissues sections at 3 dpe. Pathological scoring was carried out for two randomly selected images from each animal and by double-blind scoring on the basis of histopathological changes, including inflammation, structural changes and haemorrhage. Data in a, g-i (n = 6 hamsters per group), and k, l (n = 4 hamsters) are shown as the mean ± SEM. n = 6 mice in data b-e and n = 5 monkeys in data f. The p values (two-sided) and R2 values reflect Pearson’s correlation analysis in b-d. Significance was calculated using two-sided Mann-Whitney tests in a, g-i and using Kruskal-Wallis test with multiple comparison tests in k and l.
Supplementary information
Supplementary Information
This file contains Supplementary Figs. 1-3 and Supplementary Table 1.
Source data
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ye, T., Jiao, Z., Li, X. et al. Inhaled SARS-CoV-2 vaccine for single-dose dry powder aerosol immunization. Nature 624, 630–638 (2023). https://doi.org/10.1038/s41586-023-06809-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06809-8
This article is cited by
-
A next-generation inhalable dry powder COVID vaccine
Nature (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.