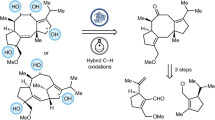
Abstract
Frustrated Lewis pairs (FLPs) are well documented for the activation of small molecules such as dihydrogen and carbon dioxide1,2,3,4. Although canonical FLP chemistry is heterolytic in nature, recent work has shown that certain FLPs can undergo single-electron transfer to afford radical pairs5. Owing to steric encumbrance and/or weak bonding association, these radicals do not annihilate one another, and they have thus been named frustrated radical pairs (FRPs). Notable preliminary results suggest that FRPs may be useful reagents in chemical synthesis6,7,8, although their applications remain limited. Here we demonstrate that the functionalization of C(sp3)–H bonds can be accomplished using a class of FRPs generated from disilazide donors and an N-oxoammonium acceptor. Together, these species undergo single-electron transfer to generate a transient and persistent radical pair capable of cleaving unactivated C–H bonds to furnish aminoxylated products. By tuning the structure of the donor, it is possible to control regioselectivity and tailor reactivity towards tertiary, secondary or primary C–H bonds. Mechanistic studies lend strong support for the formation and involvement of radical pairs in the target reaction.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data supporting the findings of this study are available within the paper and its Supplementary Information. Should any raw data files be needed in another format they are available from the corresponding author upon reasonable request.
References
Stephan, D. W. Frustrated Lewis pairs. J. Am. Chem. Soc. 137, 10018–10032 (2015).
Stephan, D. W. & Erker, G. Frustrated Lewis pair chemistry: development and perspectives. Angew. Chem. Int. Edn 54, 6400–6441 (2015).
Stephan, D. W. The broadening reach of frustrated Lewis pair chemistry. Science 354, aaf7229 (2016).
Piers, W. E., Marwitz, A. J. V. & Mercier, L. G. Mechanistic aspects of bond activation with perfluoroarylboranes. Inorg. Chem. 50, 12252–12262 (2011).
Dasgupta, A., Richards, E. & Melen, R. L. Frustrated radical pairs: insights from EPR spectroscopy. Angew. Chem. Int. Edn 60, 53–65 (2021).
Ménard, G. et al. C–H bond activation by radical ion pairs derived from R3P/Al(C6F5)3 frustrated Lewis pairs and N2O. J. Am. Chem. Soc. 135, 6446–6449 (2013).
Soltani, Y. et al. Radical reactivity of frustrated Lewis pairs with diaryl esters. Cell Rep. Phys. Sci. 1, 100016 (2020).
Aramaki, Y., Imaizumi, N., Hotta, M., Kumagai, J. & Ooi, T. Exploiting single-electron transfer in Lewis pairs for catalytic bond-forming reactions. Chem. Sci. 11, 4305–4311 (2020).
Hong, B., Luo, T. & Lei, X. Late-stage diversification of natural products. ACS Cent. Sci. 6, 622–635 (2020).
Hartwig, J. F. Evolution of C–H bond functionalization from methane to methodology. J. Am. Chem. Soc. 138, 2–24 (2016).
White, M. C. & Zhao, J. Aliphatic C–H oxidations for late-stage functionalization. J. Am. Chem. Soc. 140, 13988–14009 (2018).
Cernak, T., Dykstra, K. D., Tyagarajan, S., Vachal, P. & Krska, S. W. The medicinal chemist’s toolbox for late stage functionalization of drug-like molecules. Chem. Soc. Rev. 45, 546–576 (2016).
Golden, D. L., Suh, S.-E. & Stahl, S. S. Radical C(sp3)–H functionalization and cross-coupling reactions. Nat. Rev. Chem. 6, 405–427 (2022).
Newhouse, T. & Baran, P. S. If C–H bonds could talk: selective C–H bond oxidation. Angew. Chem. Int. Edn 50, 3362–3374 (2011).
Hartwig, J. F. & Larsen, M. A. Undirected, homogeneous C–H bond functionalization: challenges and opportunities. ACS Cent. Sci. 2, 281–292 (2016).
Carestia, A. M., Ravelli, D. & Alexanian, E. J. Reagent-dictated site selectivity in intermolecular aliphatic C–H functionalizations using nitrogen-centered radicals. Chem. Sci. 9, 5360–5365 (2018).
McMillan, A. J. et al. Practical and selective sp3 C−H bond chlorination via aminium radicals. Angew. Chem. Int. Edn 60, 7132–7139 (2021).
Saito, M. et al. N-ammonium ylide mediators for electrochemical C–H oxidation. J. Am. Chem. Soc. 143, 7859–7867 (2021).
Davies, H. M. L. & Liao, K. Dirhodium tetracarboxylates as catalysts for selective intermolecular C–H functionalization. Nat. Rev. Chem. 3, 347–360 (2019).
Chan, H. S. S., Yang, J.-M. & Yu, J.-Q. Catalyst-controlled site-selective methylene C–H lactonization of dicarboxylic acids. Science 376, 1481–1487 (2022).
Li, Y.-H., Ouyang, Y., Chekshin, N. & Yu, J.-Q. PdII-catalyzed site-selective β- and γ-C(sp3)-H arylation of primary aldehydes controlled by transient directing groups. J. Am. Chem. Soc. 144, 4727–4733 (2022).
Oeschger, R. et al. Diverse functionalization of strong alkyl C–H bonds by undirected borylation. Science 368, 736–741 (2020).
Shu, C., Noble, A. & Aggarwal, V. K. Metal-free photoinduced C(sp3)–H borylation of alkanes. Nature 586, 714–719 (2020).
Liao, K. et al. Design of catalysts for site-selective and enantioselective functionalization of non-activated primary C–H bonds. Nat. Chem. 10, 1048–1055 (2018).
Geier, S. J., Gille, A. L., Gilbert, T. M. & Stephan, D. W. From classical adducts to frustrated lewis pairs: steric effects in the interactions of pyridines and B(C6F5)3. Inorg. Chem. 48, 10466–10474 (2009).
Geier, S. J., Gilbert, T. M. & Stephan, D. W. Activation of H2 by phosphinoboranes R2PB(C6F 5)2. J. Am. Chem. Soc. 130, 12632–12633 (2008).
Wang, X. et al. One-electron oxidation of an organic molecule by B(C6F5)3; isolation and structures of stable non-para-substituted triarylamine cation radical and bis(triarylamine) dication diradicaloid. J. Am. Chem. Soc. 135, 14912–14915 (2013).
Liu, L. L., Cao, L. L., Shao, Y., Ménard, G. & Stephan, D. W. A radical mechanism for frustrated Lewis pair reactivity. Chem 3, 259–267 (2017).
Merk, A. et al. Single-electron transfer reactions in frustrated and conventional silylium ion/phosphane Lewis pairs. Angew. Chem. Int. Edn 57, 15267–15271 (2018).
Liu, L. L., Cao, L. L., Zhu, D., Zhou, J. & Stephan, D. W. Homolytic cleavage of peroxide bonds via a single electron transfer of a frustrated Lewis pair. Chem. Commun. 54, 7431–7434 (2018).
Holtrop, F. et al. Single-electron transfer in frustrated Lewis pair chemistry. Angew. Chem. Int. Edn 59, 22210–22216 (2020).
Holtrop, F. et al. Photoinduced and thermal single-electron transfer to generate radicals from frustrated Lewis pairs. Chem. Eur. J. 26, 9005–9011 (2020).
Dasgupta, A. et al. Site-selective Csp3–Csp/Csp3–Csp2 cross-coupling reactions using frustrated Lewis pairs. J. Am. Chem. Soc. 143, 4451–4464 (2021).
Jiang, H. & Studer, A. Chemistry with N-centered radicals generated by single-electron transfer-oxidation using photoredox catalysis. CCS Chem. 1, 38–49 (2019).
Kärkäs, M. D. Photochemical generation of nitrogen-centered amidyl, hydrazonyl, and imidyl radicals: methodology developments and catalytic applications. ACS Catal. 7, 4999–5022 (2017).
Brand, J. C., Roberts, B. P. & Winter, J. N. Trialkylsilylaminyl radicals. Part 1. Electron spin resonance studies of the photolysis of silylated hydrazines, hydroxylamines, triazenes, and tetrazenes. J. Chem. Soc. Perkin Trans. 2 1983, 261–270 (1983).
Cook, M. D., Roberts, B. P. & Singh, K. Silylaminyl radicals. Part 2. Free radical chain halogenation of hydrocarbons using N-halogenobis(trialkylsilyl)amines. J. Chem. Soc. Perkin Trans. 2 1983, 635–643 (1983).
Siu, J. C. et al. Electrochemical azidooxygenation of alkenes mediated by a TEMPO–N3 charge-transfer complex. J. Am. Chem. Soc. 140, 12511–12520 (2018).
Nutting, J. E., Rafiee, M. & Stahl, S. S. Tetramethylpiperidine N-oxyl (TEMPO), phthalimide N-oxyl (PINO), and related N-oxyl species: electrochemical properties and their use in electrocatalytic reactions. Chem. Rev. 118, 4834–4885 (2018).
Chateauneuf, J., Lusztyk, J. & Ingold, K. U. Absolute rate constants for the reactions of some carbon-centered radicals with 2,2,6,6-tetramethylpiperidine-N-oxyl. J. Org. Chem. 53, 1629–1632 (1988).
Romero, K. J., Galliher, M. S., Pratt, D. A. & Stephenson, C. R. J. Radicals in natural product synthesis. Chem. Soc. Rev. 47, 7851–7866 (2018).
Vasilopoulos, A., Krska, S. W. & Stahl, S. S. C(sp3)–H methylation enabled by peroxide photosensitization and Ni-mediated radical coupling. Science 372, 398–403 (2021).
Galeotti, M., Salamone, M. & Bietti, M. Electronic control over site-selectivity in hydrogen atom transfer (HAT) based C(sp3)–H functionalization promoted by electrophilic reagents. Chem. Soc. Rev. 51, 2171–2223 (2022).
Schämann, M. & Schäfer, H. J. Alkoxyamines by reaction of 2,2,6,6-tetramethylpiperidine-1-oxoammonium tetrafluoroborate with enolates. Synlett 2004, 1601–1603 (2004).
Zhang, B. & Studer, A. Stereoselective radical azidooxygenation of alkenes. Org. Lett. 15, 4548–4551 (2013).
Zhu, Q., Gentry, E. C. & Knowles, R. R. Catalytic carbocation generation enabled by the mesolytic cleavage of alkoxyamine radical cations. Angew. Chem. Int. Edn 55, 9969–9973 (2016).
Kielty, P., Farràs, P., Smith, D. A. & Aldabbagh, F. 2-(Fluoromethyl)-4,7-dimethoxy-1-methyl-1H-benzimidazole. MolBank 2020, M1129 (2020).
Studer, A. Tin-free radical chemistry using the persistent radical effect: alkoxyamine isomerization, addition reactions and polymerizations. Chem. Soc. Rev. 33, 267–273 (2004).
Norcott, P. L. et al. TEMPO-Me: an electrochemically activated methylating agent. J. Am. Chem. Soc. 141, 15450–15455 (2019).
Nelson, H. M. et al. Isolation and X-ray crystal structure of an electrogenerated TEMPO–N3 charge-transfer complex. Org. Lett. 23, 454–458 (2021).
Acknowledgements
Financial support was provided by the National Institutes of Health (R01GM134088 to S.L. and F32GM142264 to M.J.) and Genentech. We thank R. Mandel for assisting in setting up reactions with high-pressure ethane; I. Keresztes for his help in structural elucidation of complex products; J. H. Freed and A. L. Lai for their help in EPR experiments; D. B. Collum, Y. Ma and R. Woltornist for suggestions on synthesis of lithium disilazides; P. Yu for reproducing experiments; and D. Stephan for discussion. We thank the Advanced Electron-Spin Resonance Spectroscopy (ACERT) centre (NIH grant number 1R24GM146107) for providing EPR facilities.
Author information
Authors and Affiliations
Contributions
S.L. supervised the project. Z.L., M.J. and S.L. conceived the work. Z.L. and M.J. designed the experiments. Z.L., M.J., J.M.M. and J.I.M.A. conducted synthetic experiments and mechanistic studies. Y.W. conducted DFT calculations. E.V. and J.A.T. provided guidance on the project. Z.L., M.J. and S.L. wrote the paper. J.M.M., J.I.M.A., Y.W., E.V. and J.A.T. assisted in writing and editing the paper.
Corresponding author
Ethics declarations
Competing interests
A US provisional patent (no. 63/369,353) was filed on the reaction method.
Peer review
Peer review information
Nature thanks Rebecca Melen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lu, Z., Ju, M., Wang, Y. et al. Regioselective aliphatic C–H functionalization using frustrated radical pairs. Nature 619, 514–520 (2023). https://doi.org/10.1038/s41586-023-06131-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-06131-3
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.