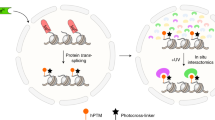
Abstract
Interactions between biomolecules underlie all cellular processes and ultimately control cell fate. Perturbation of native interactions through mutation, changes in expression levels or external stimuli leads to altered cellular physiology and can result in either disease or therapeutic effects1,2. Mapping these interactions and determining how they respond to stimulus is the genesis of many drug development efforts, leading to new therapeutic targets and improvements in human health1. However, in the complex environment of the nucleus, it is challenging to determine protein–protein interactions owing to low abundance, transient or multivalent binding and a lack of technologies that are able to interrogate these interactions without disrupting the protein-binding surface under study3. Here, we describe a method for the traceless incorporation of iridium-photosensitizers into the nuclear micro-environment using engineered split inteins. These Ir-catalysts can activate diazirine warheads through Dexter energy transfer to form reactive carbenes within an approximately 10 nm radius, cross-linking with proteins in the immediate micro-environment (a process termed µMap) for analysis using quantitative chemoproteomics4. We show that this nanoscale proximity-labelling method can reveal the critical changes in interactomes in the presence of cancer-associated mutations, as well as treatment with small-molecule inhibitors. µMap improves our fundamental understanding of nuclear protein–protein interactions and, in doing so, is expected to have a significant effect on the field of epigenetic drug discovery in both academia and industry.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All relevant data are included in the manuscript and Supplementary Information. Mass spectrometry data files have been uploaded to the MassIVE proteomics database PXD038956 (ftp://massive.ucsd.edu/MSV000090929/). Sequencing data are available on the GEO database (GSE221674).
References
Scott, D. E., Bayly, A. R., Abell, C. & Skidmore, J. Small molecules, big targets: drug discovery faces the protein–protein interaction challenge. Nat. Rev. Drug Discov. 15, 533–550 (2016).
Veltman, J. A. & Brunner, H. G. De novo mutations in human genetic disease. Nat. Rev. Genet. 13, 565–575 (2012).
Campbell, R. M. & Tummino, P. J. Cancer epigenetics drug discovery and development: the challenge of hitting the mark. J. Clin. Invest. 124, 64–69 (2014).
Geri, J. B. et al. Microenvironment mapping via Dexter energy transfer on immune cells. Science 367, 1091–1097 (2020).
Ruffner, H., Bauer, A. & Bouwmeester, T. Human protein–protein interaction networks and the value for drug discovery. Drug Discov. Today 12, 709–716 (2007).
Kouzarides, T. Chromatin modifications and their function. Cell 128, 693–705 (2007).
Ganesan, A., Arimondo, P. B., Rots, M. G., Jeronimo, C. & Berdasco, M. The timeline of epigenetic drug discovery: from reality to dreams. Clin. Epigenet. 11, 174 (2019).
Schick, S. et al. Systematic characterization of BAF mutations provides insights into intracomplex synthetic lethalities in human cancers. Nat. Genet. 51, 1399–1410 (2019).
Cheng, F. et al. Comprehensive characterization of protein–protein interactions perturbed by disease mutations. Nat. Genet. 53, 342–353 (2021).
Cheng, Y. et al. Targeting epigenetic regulators for cancer therapy: mechanisms and advances in clinical trials. Signal Transduct. Target. Ther. 4, 62 (2019).
Weinberg, D. N., Allis, C. D. & Lu, C. Oncogenic mechanisms of histone H3 mutations. Cold Spring Harb. Perspect. Med. 7, a026443 (2017).
Bagert, J. D. et al. Oncohistone mutations enhance chromatin remodeling and alter cell fates. Nat. Chem. Biol. 17, 403–411 (2021).
Nacev, B. A. et al. The expanding landscape of ‘oncohistone’ mutations in human cancers. Nature 567, 473–478 (2019).
Müller, M. M. & Muir, T. W. Histones: at the crossroads of peptide and protein chemistry. Chem. Rev. 115, 2296–2349 (2015).
Vermeulen, M. & Déjardin, J. Locus-specific chromatin isolation. Nat. Rev. Mol. Cell Biol. 21, 249–250 (2020).
Van Mierlo, G. & Vermeulen, M. Chromatin proteomics to study epigenetics—challenges and opportunities. Mol. Cell. Proteomics 20, 100056 (2021).
Ciferri, C. et al. Molecular architecture of human polycomb repressive complex 2. eLife 1, e00005 (2012).
Ruthenburg, A. J. et al. Recognition of a mononucleosomal histone modification pattern by BPTF via multivalent interactions. Cell 145, 692–706 (2011).
Zhao, S., Yue, Y., Li, Y. & Li, H. Identification and characterization of ‘readers’ for novel histone modifications. Curr. Opin. Chem. Biol. 51, 57–65 (2019).
Burton, A. J. et al. In situ chromatin interactomics using a chemical bait and trap approach. Nat. Chem. 12, 520–527 (2020).
Kleiner, R. E., Hang, L. E., Molloy, K. R., Chait, B. T. & Kapoor, T. M. A chemical proteomics approach to reveal direct protein–protein interactions in living cells. Cell Chem. Biol. 25, 110–120 (2018).
Seath, C. P., Trowbridge, A. D., Muir, T. W. & MacMillan, D. W. C. Reactive intermediates for interactome mapping. Chem. Soc. Rev. 50, 2911–2926 (2021).
Villaseñor, R. et al. ChromID identifies the protein interactome at chromatin marks. Nat. Biotechnol. 38, 728–736 (2020).
Ummethum, H. & Hamperl, S. Proximity labeling techniques to study chromatin. Front. Genet. 11, 450 (2020).
Baldi, S., Korber, P. & Becker, P. B. Beads on a string—nucleosome array arrangements and folding of the chromatin fiber. Nat. Struct. Mol. Biol. 27, 109–118 (2020).
Stevens, A. J. et al. A promiscuous split intein with expanded protein engineering applications. Proc. Natl Acad. Sci. USA 114, 8538–8543 (2017).
Stevens, A. J. et al. Design of a split intein with exceptional protein splicing activity. J. Am. Chem. Soc. 138, 2162–2165 (2016).
Scott, W. A. & Campos, E. I. Interactions with histone H3 & tools to study them. Front. Cell Dev. Biol. 8, 701 (2020).
Pan, D. et al. Mechanism of centromere recruitment of the CENP-A chaperone HJURP and its implications for centromere licensing. Nat. Commun. 10, 4046 (2019).
Chen, C. C. et al. Establishment of centromeric chromatin by the CENP-A assembly factor CAL1 requires FACT-mediated transcription. Dev. Cell 34, 73–84 (2015).
Bennett, R. L. et al. A mutation in histone H2B represents a new class of oncogenic driver. Cancer Discov. 9, 1438–1451 (2019).
McGinty, R. K. & Tan, S. Nucleosome structure and function. Chem. Rev. 115, 2255–2273 (2015).
McBride, M. J. et al. The nucleosome acidic patch and H2A ubiquitination underlie mSWI/SNF recruitment in synovial sarcoma. Nat. Struct. Mol. Biol. 27, 836–845 (2020).
Dao, H. T., Dul, B. E., Dann, G. P., Liszczak, G. P. & Muir, T. W. A basic motif anchoring ISWI to nucleosome acidic patch regulates nucleosome spacing. Nat. Chem. Biol. 16, 134–142 (2020).
Skrajna, A. et al. Comprehensive nucleosome interactome screen establishes fundamental principles of nucleosome binding. Nucleic Acids Res. 48, 9415–9432 (2020).
Anink-Groenen, L. C. M., Maarleveld, T. R., Verschure, P. J. & Bruggeman, F. J. Mechanistic stochastic model of histone modification pattern formation. Epigenetics Chromatin 7, 30 (2014).
Tachiwana, H. et al. Chromatin structure-dependent histone incorporation revealed by a genome-wide deposition assay. eLife 10, e66290 (2021).
Grandi, F. C., Modi, H., Kampman, L. & Corces, M. R. Chromatin accessibility profiling by ATAC-seq. Nat. Protoc. 17, 1518–1552 (2022).
Xu, T. et al. Structure of nucleosome-bound DNA methyltransferases DNMT3A and DNMT3B. Nature 586, 151–155 (2020).
Bolton, M. A. et al. Aurora B kinase exists in a complex with survivin and INCENP and its kinase activity is stimulated by survivin binding and phosphorylation. Mol. Biol. Cell 13, 3064–3077 (2002).
Hodges, C., Kirkland, J. G. & Crabtree, G. R. The many roles of BAF (mSWI/SNF) and PBAF complexes in cancer. Cold Spring Harb. Perspect. Med. 6, a026930 (2016).
Filippakopoulos, P. et al. Selective inhibition of BET bromodomains. Nature 468, 1067–1073 (2010).
Shi, J. & Vakoc, C. R. The mechanisms behind the therapeutic activity of BET bromodomain inhibition. Mol. Cell 54, 728–736 (2014).
Savitski, M. M. et al. Multiplexed proteome dynamics profiling reveals mechanisms controlling protein homeostasis. Cell 173, 260–274 (2018).
Stein, E. M. et al. The DOT1L inhibitor pinometostat reduces H3K79 methylation and has modest clinical activity in adult acute leukemia. Blood 131, 2662–2669 (2018).
Gilan, O. et al. Functional interdependence of BRD4 and DOT1L in MLL leukemia. Nat. Struct. Mol. Biol. 23, 673–681 (2016).
Osman, S. & Cramer, P. Structural biology of RNA polymerase II transcription: 20 years on. Annu. Rev. Cell Dev. Biol. 36, 1–34 (2020).
Cramer, P. Organization and regulation of gene transcription. Nature 573, 45–54 (2019).
Young, R. A. RNA polymerase II. Annu. Rev. Biochem. 60, 689–715 (1991).
Santo, L. et al. AT7519, a novel small molecule multi-cyclin-dependent kinase inhibitor, induces apoptosis in multiple myeloma via GSK-3Β activation and RNA polymerase II inhibition. Oncogene 29, 2325–2336 (2010).
Harlen, K. M. & Churchman, L. S. The code and beyond: transcription regulation by the RNA polymerase II carboxy-terminal domain. Nat. Rev. Mol. Cell Biol. 18, 263–273 (2017).
Trowbridge, A. D. et al. Small molecule photocatalysis enables drug target identification via energy transfer. Proc. Natl Acad. Sci. USA 119, e2208077119 (2022).
Cox, J. & Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 26, 1367–1372 (2008).
Tyanova, S. et al. The Perseus computational platform for comprehensive analysis of (prote)omics data. Nat. Methods 13, 731–740 (2016).
Traube, F. R. et al. Isotope-dilution mass spectrometry for exact quantification of noncanonical DNA nucleosides. Nat. Protoc. 14, 283–312 (2019).
Zhou, Y. et al. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 10, 1523 (2019).
Babicki, S. et al. Heatmapper: web-enabled heat mapping for all. Nucleic Acids Res. 44, 147–153 (2016).
Acknowledgements
Research reported in this publication was supported by the NIH National Institute of General Medical Sciences (R35GM134897-01, R01-GM103558-03 and R37-GM086868) and the NIH National Cancer Institute (P01 CA196539). A.J.B. was a Damon Runyon Fellow of the Damon Runyon Cancer Research Foundation (DRG-2283-17). We thank S. Kyin and H. H. Shwe at the Princeton Proteomics Facility. The mock volcano plot in Fig. 1 was created using BioRender.
Author information
Authors and Affiliations
Contributions
C.P.S., A.J.B., T.W.M. and D.W.C.M. conceived the work. C.P.S., A.J.B., X.S., G.L., R.E.K., T.W.M. and D.W.C.M. designed and executed the experiments. C.P.S., A.J.B., T.W.M. and D.W.C.M. prepared this manuscript.
Corresponding authors
Ethics declarations
Competing interests
A provisional US patent has been filed by D.W.C.M. and C.P.S. on the basis of photocatalysts used in this work, 62/982,366 and 63/076,658, international application no. PCT/US2021/019959. D.W.C.M. declares an ownership interest and C.P.S. declares an affiliation interest in the company Dexterity Pharma LLC, which has commercialized materials used in this work. The remaining authors declare no competing interests.
Peer review
Peer review information
Nature thanks Stephen Frye, Michiel Vermeulen and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Supporting data for H3.1 vs CENP-A and H2A WT vs H2A E92K experiments.
Anti-FLAG western blot for the expression of H3.1-HA-CfaN-FLAG, H3.1 (4xAla)-HA-CfaN-FLAG (not used in MS experiment) and CENP-A-HA-CfaN-FLAG. H4 visualized by coomassie blue stain is provided as a loading control. MW of H3.1-HA-Cfa-N-FLAG = 29,365 Da. B) GO analysis of proteins enriched in H3.1 samples. Data shows significant GO terms related to Chromatin organization, remodelling, and epigenetic control of gene expression. Analysis performed using metascape. C) Anti-FLAG western blot for the expression of H2A-HA-CfaN-FLAG and H2A-E92K-HA-CfaN-FLAG. H4 visualized by coomassie blue stain is provided as a loading control. D) In-nuclei splicing reactions for H2A (WT) and H2A (E92K) constructs in the presence of CfaC-Ir (referred to as Int) and control as visualized by anti-HA and anti-FLAG western blot. H4 visualized by coomassie blue stain is provided as a loading control. MW of H2A-HA-CfaN-FLAG = 28,121 Da. For blot source data, see Supplementary Fig. 1.
Extended Data Fig. 2 Interactome of acidic patch triple mutant and comparison to H2A E92K.
A) Left: Anti-FLAG western blot for the expression of H2A-HA-CfaN-FLAG and H2A-3xAla-HA-CfaN-FLAG. Note, 3xAla refers to the acidic patch mutations E61A, E90A and E92A in H2A. Right: In-nuclei splicing reactions for H2A (WT) and H2A (3xAla) constructs in the presence of CfaC-Ir as visualized by anti-HA and anti-FLAG western blot. B) Graphic representing a comparative experiment between H2A (WT) and H2A (3xAla). C) Volcano plot derived from a two-sided t-test showing proteins enriched in a comparative proteomics study between H2A (WT) and H2A (3xAla) using the µMap workflow. Proteins are highlighted that represent acidic patch binders and chromatin remodelers, and are colour coded according to the remodelling complex they belong to. FDR values were calculated using the Benjamini–Hochberg procedure, as described in Methods. D) Table showing known chromatin remodelling complexes that are enriched in the µMap dataset between H2A (WT) and H2A (3xAla). Proteins are colour coded by enrichment. Blue = Log2FC >0.5, FDR <0.05. Cyan = Meets FDR requirement, FDR <0.05. Red = Not enriched. Black = Not in dataset. Data shows that nucleosome binding for most (80%) of all complex members is affected by the deletion of the H2A acidic patch. E) Venn diagram showing overlap in the loss of function proteins in the H2A (WT) vs H2A (E92K) and H2A (WT) vs H2A (3xAla) datasets. Approximately two thirds of the hits (Log2FC >0.5, FDR <0.05) observed in the H2A (E92K) experiment are also hits in the H2A (3xAla) experiment. For blot source data, see Supplementary Fig. 1.
Extended Data Fig. 3 Measurement of relative quantities of transfected histone H3.1.
A) Representative western blot showing relative concentrations of native H3 and transfected H3.1-HA-CfaN-FLAG. B) Bar graph showing relative intensities of transfected and native H3. N = 6 independent biological replicates, (**** = P < 0.0001). Over 6 replicates transfected histones make up ≈1/12 of all histone H3. For blot source data, see Supplementary Fig. 1.
Extended Data Fig. 4 Comparison of proteomics data obtained from stable cell lines.
To compare the effects of stable expression on µMap we conducted our two core workflows in HEK 293T cells expressing H2A-HA-CfaN-FLAG and/or H2A(E92K)-HA-CfaN-FLAG. A) Anti-FLAG western blot showing stable expression of H2A-HA-CfaN-FLAG and H2A(E92K)-HA-CfaN-FLAG at the indicated viral titre. Ponceau staining is provided as a loading control. B) Left: Volcano plot derived from a two-sided t-test showing µMap target ID experiment with JQ-1 in stably expressing cells with 1 µM JQ-1. Data is consistent with transiently transfected cell lines. Right: Volcano plot derived from a two-sided t-test showing µMap experiment comparing wild-type and E92K interactomes. C) Comparison of H2A (WT) vs H2A (E92K) proteomics hits from transiently transfected cells compared to stably expressing cells. Table shows the four proteins investigated in this study. D) Venn diagram showing all proteins in common between datasets (Log2FC >0.5, FDR <0.05). Overlap is observed for key chromatin modifying proteins. For blot source data, see Supplementary Fig. 1.
Extended Data Fig. 5 ATAC-Seq extended data.
A) Hierarchical clustering analysis: Read counts in merged peaks are used to calculate distances between samples and generate a heatmap of sample-to-sample relationships. Data shows strong association between replicates. B) Differential binding analysis: The R package DiffBind is used for differential region of interest (ROI) detection using FDR <= 0.05 as a cutoff. Peaks that have a fold change below zero and FDR <= 0.05 indicate regions with lower accessibility. Peaks with a fold change greater than zero and FDR <= 0.05 indicate regions with higher accessibility. C) Average ATAC-seq profile across all samples at annotated transcription start sites (TSS’s): Data shows the average profile of ATAC-seq peaks falling within +/− 3Kb of annotated TSS sites genome-wide. D) Differential peak annotation: Peaks are annotated based on genomic features they overlap. When annotations overlap, assignment priorities are Promoter > 5′UTR > 3′UTR > Exon > Intron > Downstream > Intergenic. E) Histogram of overlapping features under merged peaks, ranked by number of peaks. F) Distribution of genomic distance from peak summits to the nearest TSS.
Extended Data Fig. 6 APEX2 comparison – Validation.
Comparison of μMap to APEX approaches: A) Key experiments in this study were repeated using previously reported APEX2 methodology according to the published procedure: Hung, V., Udeshi, N., Lam, S. et al. Spatially resolved proteomic mapping in living cells with the engineered peroxidase APEX2. Nat Protoc 11, 456–475 (2016). 10.1038/nprot.2016.018. B) Western blot showing relative levels of biotinylation in the fractionated nuclear, cytosolic and membrane proteome. Data shows that the APEX construct is localized to the nucleus. C) Western blot showing data from a 9-plex experiment representing two different experiments (H2A (WT) vs. H2A (E92K) and H2A ± JQ-1; each N = 3). H2A-APEX2 expression and levels of biotinylation are even across all replicates. MW: H2A-HA-APEX2-FLAG = 41,981 Da. D) Fractionation of HEK 293T cells transfected with either H2A-APEX or H2A-CfaN shows that expressed HA-tagged histone fusion proteins are localized to the chromatin fraction. Left) Anti-HA western blot showing H2A-HA-APEX2-FLAG localization in different cellular fractions. Appropriate loading controls are provided. Right) Anti-HA western blot showing H2A-HA-CfaN-FLAG localization to different cellular fractions. Appropriate loading controls are provided. E) Volcano plots derived from a two-sided t-test showing comparison of results obtained from APEX2 proximity labelling and µMap experiments for H2A (WT) vs H2A (E92K). F) Model for interactomes detected using APEX2 and Iridium conjugated nucleosomes. The short radius of µMap only allows labelling of proteins directly interacting with the expressed nucleosome. The longer radius of APEX2 leads to additional labelling of the wild-type nucleosomal interactomes that border the expressed histone, leading to significant reduction in signal to noise. For blot source data, see Supplementary Fig. 1.
Extended Data Fig. 7 Supporting data for H2A ± JQ-1 and Pinometostat.
A) In nucleo splicing reactions for H2A-CfaN constructs in the presence of CfaC-Ir as visualized by anti-HA/anti-FLAG western blot. Left (untreated cells), middle (cells treated with JQ-1), right (cells treated with Pinometostat). MW of H2A-HA-CfaN-FLAG = 28,121 Da. B) Treatment of HEK 293T cells with pinometostat for 24 h (0, 0.5, 2.5 μM) results in ≈60% decrease in H3K79 dimethylation, as visualized by western blotting with an anti-H3K79me2 antibody. Histones H3, H2A, and H2B, stained by coomassie blue, are provided as a loading control. C) Volcano plots derived from a two-sided t-tests comparing APEX2 target ID proximity labelling to µMap target ID for H2A ± JQ-1. D) Volcano plot derived from a two-sided t-test for comparison of H2A ± JQ-1 at two different treatment concentrations (5 µM (left) and 1 µM (right)). E) Chart comparing the observed log2 (fold change) values returned for BRD2/3/4 at the indicated treatment concentrations. For blot source data, see Supplementary Fig. 1.
Extended Data Fig. 8 RNA-Seq data.
A) The global transcriptional change across the groups (WT vs JQ-1) compared was visualized by a volcano plot derived from a two-sided t-test. Each data point in the scatter plot represents a gene. The log2 fold change of each gene is represented on the x-axis and the log10 of its adjusted p-value is on the y-axis. Genes with an adjusted p-value less than 0.05 and a log2 fold change greater than 1 are indicated by red dots. These represent up-regulated genes. Genes with an adjusted p-value less than 0.05 and a log2 fold change less than −1 are indicated by blue dots. These represent down-regulated genes. B) A bi-clustering heatmap was used to visualize the expression profile of the top 30 differentially expressed genes (WT vs JQ-1) sorted by their adjusted p-value by plotting their log2 transformed expression values in samples. This analysis is useful to identify co-regulated genes across the treatment conditions. C&D) The same analysis was performed on (WT vs Pinometostat) samples. E) Table showing RNA-seq fold changes and P values for proteomic hits in from H2A vs H2A +JQ-1 experiment. Data strongly suggests that enrichment is due to proximity and not global changes in gene expression. F) Table showing RNA-seq fold changes and P values for proteomic hits in from H2A vs H2A + Pinometostat experiment. Data strongly suggests that enrichment is due to proximity and not global changes in gene expression Importantly, genes enriched in proteomic analysis were NOT enriched in RNA-seq, showing enrichment is based on proximity, rather than changes in gene expression caused by ligand treatment.
Extended Data Fig. 9 Validation of RBP1 expression and splicing.
Left: Anti-HA western blot for the expression of RBP1-HA-CfaN-FLAG. H4 visualized by coomassie blue stain is provided as a loading control. Middle: In nucleo splicing reactions for RBP1-HA-CfaN-FLAG in the presence of CfaC-biotin (+) as visualized by streptavidin-800. H4 visualized by coomassie blue stain is provided as a loading control. Right: Labelling of nuclear proteins after installation of Ir photocatalyst (45 and 90 s irradiation with blue LEDs) versus free Ir control as visualized by streptavidin-800. MW of RBP1-HA-CfaN-FLAG = 231,290 Da. For blot source data, see Supplementary Fig. 1.
Extended Data Fig. 10 Volcano plots of all proteomics experiments analysed with two unique peptide filter.
Volcano plots derived from a two-sided t-tests for MS experiments. All proteins presented require two unique peptides found across all replicates. Cut-offs are Log2FC >0.5 and FDR <0.05. For blot source data, see Supplementary Fig. 1.
Supplementary information
Supplementary Information
Supplementary Figs. 1–3, Tables 1 and 2 and synthetic procedures.
Supplementary Table 3
Processed proteomics data for all experiments.
Supplementary Table 4
Protein group data for all MS experiments.
Supplementary Table 5
Peptide groups data for all MS experiments.
Supplementary Table 6
ATAC-seq differentially expressed genes.
Supplementary Table 7
RNA-seq differentially expressed genes.
Supplementary Table 8
Plasmid sequences used in the study.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Seath, C.P., Burton, A.J., Sun, X. et al. Tracking chromatin state changes using nanoscale photo-proximity labelling. Nature 616, 574–580 (2023). https://doi.org/10.1038/s41586-023-05914-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-05914-y
This article is cited by
-
Bioorthogonal photocatalytic proximity labeling in primary living samples
Nature Communications (2024)
-
Interrogating epigenetic mechanisms with chemically customized chromatin
Nature Reviews Genetics (2024)
-
Metal complex catalysts broaden bioorthogonal reactions
Science China Chemistry (2024)
-
Proximity labelling to study chromatin interactomes
Nature Reviews Cancer (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.