Abstract
Tropical peatlands cycle and store large amounts of carbon in their soil and biomass1,2,3,4,5. Climate and land-use change alters greenhouse gas (GHG) fluxes of tropical peatlands, but the magnitude of these changes remains highly uncertain6,7,8,9,10,11,12,13,14,15,16,17,18,19. Here we measure net ecosystem exchanges of carbon dioxide, methane and soil nitrous oxide fluxes between October 2016 and May 2022 from Acacia crassicarpa plantation, degraded forest and intact forest within the same peat landscape, representing land-cover-change trajectories in Sumatra, Indonesia. This allows us to present a full plantation rotation GHG flux balance in a fibre wood plantation on peatland. We find that the Acacia plantation has lower GHG emissions than the degraded site with a similar average groundwater level (GWL), despite more intensive land use. The GHG emissions from the Acacia plantation over a full plantation rotation (35.2 ± 4.7 tCO2-eq ha−1 year−1, average ± standard deviation) were around two times higher than those from the intact forest (20.3 ± 3.7 tCO2-eq ha−1 year−1), but only half of the current Intergovernmental Panel on Climate Change (IPCC) Tier 1 emission factor (EF)20 for this land use. Our results can help to reduce the uncertainty in GHG emissions estimates, provide an estimate of the impact of land-use change on tropical peat and develop science-based peatland management practices as nature-based climate solutions.
Similar content being viewed by others
Main
Over the Holocene, tropical peatlands have accumulated at least 75 Gt of carbon (C) in partially decomposed debris (wood, roots, litter, leaves) under waterlogged anoxic environments1,2,3,4,5. A fine balance between hydrology, ecology and landscape morphology has resulted in this long-term C store6,7,8. Climate and other environmental changes are, however, affecting this C store as a result of warming, drying conditions and change in disturbance rates9,10,11,12,13,14,15,16,17. Particularly, decreased rainfall, increased seasonality and frequent days without rainfall are resulting in GWL drawdowns7,12,13, which cause C loss14,15,16,17.
Tropical peatlands are among the world’s most threatened ecosystems owing to land demand driven by population growth and economic development21. In Southeast Asia, which hosts at least one-third of the total tropical peatlands3,4, most peatland conversion has occurred since the late 1990s21. A total peatland area of 7.8 million hectares is managed for agriculture and silviculture, of which more than one million hectares are under fibre wood (mostly A. crassicarpa) plantations21. Artificial GWL drawdown in agriculture and plantations on peatland exposes previously accumulated peat organic material to oxygen and promotes aerobic decomposition of organic C, resulting in carbon dioxide (CO2) emissions22,23 and associated land subsidence24,25,26. At present, the IPCC Tier 1 CO2 EF20 for short-rotation tree plantations on tropical peat is entirely based on the use of short-term measurements from the 3–8 years after drainage using subsidence22 and soil-chamber23 techniques. Furthermore, tropical peatlands emit methane (CH4)27,28 and nitrous oxide (N2O)17,29, potent GHGs, yet assessments of the contributions made by these gases to the full peatland GHG balance are scarce20. Existing estimates of GHG emissions from tropical peatlands continue to be debated20,30 with large observed variability (0.04–2.79 GtCO2-eq year−1)18 and resulting uncertainty19.
From a climate-forcing perspective, the effects of a land-use change on the atmospheric GHG concentrations (that is, the extra GHG fluxes that the atmosphere will see because of current land use) will be determined by the change in emissions relative to those occurring before the land-use change17,31. Despite the increasing awareness of the significance of GHG fluxes from managed peatlands, there have been few experimental studies evaluating the GHG balance before and after a land-use change has occurred. Thus, a better quantitative and process-based knowledge of how the tropical peat C store responds to land-use change under current climate conditions is an urgent area of enquiry that can inform strategies for responsible peatland management32 under national and global frameworks of climate change.
This study represents, to the best of our knowledge, the first GHG balance investigation undertaken in any fibre wood plantation on peatland (and indeed any soil type) globally to cover a full plantation rotation and all major GHG flux terms, including biomass C loss owing to plantation establishment, C export in harvested wood and fluvial C exports. We compare the GHG balance at the Acacia plantation with more than 5 years of measurements at the degraded site and 5 years of measurements at the intact site (Figs. 1 and 2 and Extended Data Table 1).
a, Location of research sites with satellite images from Landsat 8 (source: https://earthexplorer.usgs.gov/). Photographs of the eddy covariance instruments installed at the top of the tower at Acacia plantation (b), degraded site (c) and intact site (d). For detailed site information, see Methods. Integrated eddy covariance footprint contour lines from 10% to 80% in 10% intervals over Acacia plantation for October 2016–May 2021 (e), degraded site for October 2016–May 2022 (f) and intact site for June 2017–May 2022 (g). GWL, peat subsidence, oxidative peat decomposition, soil N2O flux and soil-sampling locations at Acacia plantation (h), intact site (i) and degraded site (j). An integrated climatologic footprint analysis indicated that approximately 80% of fluxes originated within 1,000 m in the upwind direction of each tower. Esri, HERE, Garmin, (c) OpenStreetMap contributors and the GIS user community.
To quantify total GHG balance in carbon dioxide equivalent (CO2-eq), we used a sustained-flux global-warming potential (SGWP) of 1, 45 and 270 for CO2, CH4 and N2O, respectively, over a 100-year time period39. Total GHG balance = (net ecosystem CO2 exchange + net ecosystem CH4-C exchange + fluvial C export + C export in harvested wood, where applicable) + (net ecosystem CH4 exchange × SGWP) + (soil N2O flux × SGWP). We assumed that all fluvial C export is ultimately converted to CO2 (ref. 38). Avoided emissions from bioenergy production are calculated by assuming that 54% of harvested wood is used for bioenergy production (details in Supplementary Methods). The bold numbers indicate net impact of land-use change. Positive values indicate emission to the atmosphere and negative values indicate avoided emission.
CO2 flux
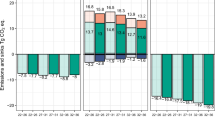
Over a 4.7-year period encompassing one full Acacia plantation rotation (the fourth rotation during 17–22 years after drainage), the average GWL was −0.65 ± 0.17 m, for which a negative GWL indicates that the water level was below the peat surface (Extended Data Table 2). Net ecosystem exchanges of CO2 (NEE-CO2; net gaseous CO2 exchange between ecosystem and atmosphere) varied with plantation age; it was highest (48.4 ± 4.7 tCO2 ha−1 year−1) in the first year after planting, lowest (−8.8 ± 4.5 tCO2 ha−1 year−1) in the third year with highest tree growth and then rose again to 11.7 ± 6.0 tCO2 ha−1 year−1 before harvesting (Extended Data Fig. 1 and Extended Data Table 3) (for which positive NEE-CO2 values indicate net CO2 emissions and negative values indicate net CO2 uptake). The substantial net CO2 emissions during the early stage of the plantation were mainly because of the low photosynthetic rates of the young trees and also potentially driven by the decomposition of organic matter from harvested residues (that is, leaves, branches, bark, roots and stumps) from the previous plantation rotation. After canopy closure, the emissions from oxidative peat decomposition (Extended Data Table 3) were largely outbalanced by high rates of photosynthesis and C fixation (Extended Data Fig. 1). Over the plantation rotation, the average NEE-CO2 was 9.5 ± 4.5 tCO2 ha−1 year−1 (Fig. 2 and Extended Data Table 4). The average peat subsidence rate was −3.0 ± 0.9 cm year−1 (Extended Data Table 5) (negative peat subsidence indicates that the ground surface elevation was falling). The C export in harvested wood was 26.3 ± 1.4 tC ha−1, corresponding to 96.5 ± 5.2 tCO2 ha−1 (20.5 ± 1.1 tCO2 ha−1 year−1 when annualized over the plantation rotation; Fig. 2 and Extended Data Table 4). Thus, the sum of NEE-CO2 and C export in harvested wood indicates that the Acacia plantation functioned as a CO2 source of 30.0 ± 4.6 tCO2 ha−1 year−1 over the plantation rotation (Fig. 2 and Extended Data Table 4). The observed net CO2 emissions can be attributed to peat aeration owing to a consistently deep GWL, which enhances heterotrophic respiration rates, combined with a higher soil temperature (intact site = 27.5 ± 0.5 °C versus Acacia site = 29.3 ± 1.0 °C; Extended Data Table 2) owing to both canopy-cover loss and GWL drawdown, which further boosts microbial activities and heterotrophic respiration. Note that this calculation conservatively assumed that all harvested C would be returned to the atmosphere as CO2, as harvested wood was used to produce bioenergy and pulp products, which is common practice for these types of forest plantation.
We also calculated avoided emissions of 7.3 ± 0.4 tCO2-eq ha−1 year−1, resulting from the use of tree biomass for bioenergy (see Supplementary Methods), in place of coal burning that would otherwise have been used to support pulp mill operations (Fig. 2 and Extended Data Table 4). This avoided emission through bioenergy production as a by-product of the pulp manufacturing process could be considered to partly offset emissions from the plantation itself, although it clearly does not negate the peat CO2 emission.
In degraded peat swamp forest, the GWL was consistently low throughout the study period at the degraded site, with an average of −0.69 ± 0.18 m (Extended Data Table 2). NEE-CO2 did not show clear seasonal and interannual variability. The degraded site emitted 40.8 ± 4.4 tCO2 ha−1 year−1 and subsided −3.6 ± 1.2 cm year−1 (Fig. 2 and Extended Data Table 3), consistent with previous observations in ref. 17. The observed large CO2 emissions can be attributed to peat aeration owing to a consistently deep GWL as described for the Acacia site. Coarse woody debris from fallen dead trees may also have contributed to the CO2 emissions, as fallen trees do not decompose instantaneously, providing a lagged but sustained contribution to CO2 emissions.
In intact peat swamp forest, the GWL followed the seasonal and interannual variability in rainfall (Extended Data Table 2), in line with the initial measurements in ref. 17. The GWL remained below the peat surface for >80% of the study period, indicating that a substantial part of the upper peat profile was aerated. NEE-CO2 showed strong seasonal and interannual patterns corresponding to the GWL fluctuation (Extended Data Fig. 1). The results indicate that large net CO2 emissions during dry seasons were not entirely balanced by relatively small net CO2 uptake during the wet seasons (Extended Data Fig. 1). Over a 5-year measurement period, the annual NEE-CO2 ranged from 9.1 ± 3.7 to 25.6 ± 4.1 tCO2 ha−1 year−1, with an average value of 15.3 ± 3.7 tCO2 ha−1 year−1 (Extended Data Table 3). The CO2 emissions owing to GWL drawdown are consistent with previous studies in tropical peatlands in which reduced peat accumulation rates8,15, a hiatus in peat genesis33 or even C loss14,15,16,17 have been reported in response to droughts driven by intense and frequent El Niño–Southern Oscillation activity.
The evapotranspiration measurements clearly indicate that actual daily evapotranspiration (4.2 mm day−1) exceeded daily rainfall for around 76% of the study period (Extended Data Table 2). Notably, we observed more than 220 days without rainfall every year (Extended Data Table 2). During days without rainfall, the GWL would recede at an average rate of 10.3 mm day−1, resulting from the seepage and evapotranspiration in this ombrotrophic environment. The seepage rates owing to groundwater and subsurface flows, as calculated from GWL drawdown between midnight and 06:00 local time (when evapotranspiration is negligible; Extended Data Fig. 2), was 1.4 mm during a single 12-h night (that is, 2.8 mm day−1), which is similar to a pristine tropical peatland in Brunei7. The evapotranspiration resulted in GWL drawdown of 7.5 mm day−1 during days without rainfall at our study site. During prolonged drought periods induced by climate extremes in 2019, when we observed only 45 mm rainfall during a consecutive 90-day period, the GWL fell to below −0.70 m, resulting in a large peat surface drop of −7.0 ± 1.3 cm in the intact sites (Extended Data Table 5). The peat surface had not rebounded from the 2019 perturbation by the end of the record, resulting in a total subsidence of −7.1 ± 2.4 cm during the period December 2017–May 2022 (Extended Data Table 5).
The close link between net rainfall (total rainfall minus evapotranspiration) and GWL (Extended Data Fig. 2) confirms that observed relatively low rainfall combined with increased seasonality and days without rainfall play a central role in shaping the seasonal and interannual variability of intact tropical peatland hydrology and therefore of CO2 fluxes7. The El Niño34 and positive Indian Ocean Dipole (IOD)35 observations indicate that the region has experienced several moderate to very strong drought events in the recent past, suggesting that tropical peatland ecosystems are exposing and responding to changes in rainfall regime, which may limit their role as a carbon sink.
Given that GWLs at our intact forest site were slightly lower (annual rainfall = 1,883 mm year−1; Extended Data Table 2) than those reported for a pristine peat swamp forest (annual rainfall = 2,880 mm year−1)7, we cannot entirely rule out some impact of surrounding land use on the hydrology and function of the peat dome as a whole. However, plantation water management is not believed to have affected forest hydrology at the flux tower footprint in the study area. Previous analysis suggested that such effects occurred within 300 m of the plantation boundary25, and recent multivariate analysis indicates that subsidence in the interior forest is independent of distance from plantation canals26. This is further indicated by subsidence at rates of −1.4 cm year−1 (Extended Data Table 5) observed at sampling locations between 7 to 10 km from the active plantation edge (Fig. 1). There was clearly a strong association during the study period between C loss, subsidence and droughts driven by regional climate extremes26. Our results indicate that even low-level or indirect human disturbance (for example, by means of climate change) can lead to C loss, highlighting the hydroclimatic vulnerability of C in forested tropical peatlands13,14,15,16.
Other GHG fluxes and C loss
Net ecosystem exchanges of CH4 (NEE-CH4; net gaseous CH4 exchange between ecosystem and atmosphere) were positive at all sites, but lower in the Acacia plantation and degraded site than in the intact site (Fig. 2 and Extended Data Table 4), consistent with lower GWLs promoting methanotrophy in the aerobic zone27. GWL drawdown below the root zone will also limit plant-mediated transport of CH4 from the anaerobic zone to the atmosphere27. The finding that CH4 emissions remained positive despite low GWLs (Fig. 3) may be attributed to emissions from vegetation and water surfaces27.
a, Carbon dioxide (CO2). b, Methane (CH4). Relationship between net CO2 and CH4 fluxes and GWL were derived from published eddy covariance flux studies in tropical peatlands. The solid lines show the best-fit models and the dashed lines show 95% confidence intervals. The statistical test used a significance level of 5%. Positive values indicate net emission to the atmosphere, negative values indicates net uptake by the ecosystem. CO2 results are compared with a previous relationship between CO2 fluxes and GWL derived from subsidence22 and soil flux chamber23 measurements and a relationship for peatlands in the United Kingdom and Ireland45 is based on eddy covariance measurements. Positive and negative GWL values indicate the water level above and below the peat hollow surface, respectively.
Soil N2O fluxes at the Acacia plantation were higher than at the degraded and intact sites (Fig. 2 and Extended Data Fig. 3), but were within the range of fluxes reported from oil palm plantation on peat29,30. Higher emissions from the plantation can be explained by a combination of leguminous Acacia trees that increase mineral nitrogen (N) availability through N fixation; accelerated mineralization of the peat under aerobic conditions (Extended Data Fig. 3) releasing mineral N as ammonium (Extended Data Table 1) and producing N2O and nitrate during the nitrification process; and high availability of labile C and nitrate from rapid fine-root turnover (Extended Data Table 1), providing a substrate for denitrifier heterotrophs.
A previous study36 within the same landscape reported fluvial C export of 0.3 ± 0.1 and 0.5 ± 0.1 tC ha−1 year−1 in the intact and degraded sites, respectively. Owing to lack of fluvial C-export measurements for the Acacia plantation, we used a value of 0.4 ± 0.1 tC ha−1 year−1 from a managed oil palm plantation in Southeast Asia37. Notably, fluvial C exports are fairly small compared with direct CO2 emissions. We conservatively assume that all fluvial C export is ultimately emitted as CO2 (ref. 38). The increased fluvial C export from the plantation and degraded forest may be attributed to enhanced mineralization with deeper GWL37.
Finally, the measured aboveground and belowground biomass C stock was highest in the intact forest (aboveground biomass = 105.6 ± 21.7 tC ha−1 and belowground biomass = 24.8 ± 5.1 tC ha−1) and decreased in the degraded forest (aboveground biomass = 88.7 ± 22.9 tC ha−1 and belowground biomass = 18.2 ± 4.7 tC ha−1) and the Acacia plantation (aboveground biomass = 35.2 ± 1.9 tC ha−1 and belowground biomass = 7.2 ± 0.4 tC ha−1, averaged over whole plantation rotation). Over a 100-year timescale (see Methods), biomass C losses owing to land-use change from intact forest were 0.9 ± 0.0 and 3.2 ± 0.9 tCO2 ha−1 year−1 in the degraded forest and Acacia plantation, respectively (Fig. 2 and Extended Data Table 4). Biomass C loss owing to plantation establishment on degraded forest was 2.4 ± 0.9 tCO2 ha−1 year−1 (Extended Data Table 4).
Net GHG balance of Acacia plantation
Comparison of GHG fluxes at the Acacia plantation and degraded and intact sites in this tropical peat landscape indicates that conversion of intact forest to Acacia plantation or degraded forest results in a substantial increase in CO2 and N2O emissions and a decrease in CH4 emissions. Overall, the associated warming impact of higher CO2 and N2O emissions is larger than the accompanying cooling impact of lower CH4 emissions (Fig. 2). We calculated total GHG balances of all sites using a sustained-flux global-warming potential of 1, 45 and 270 for CO2, CH4 and N2O, respectively, over a 100-year time period39. The GHG balance and the subsidence rate in the Acacia plantation were around two times higher than those measured at the intact site (Fig. 2 and Extended Data Table 5). The measured CO2 emissions in this study indicate that the long-term rate of C accumulation of 2.8 tCO2 ha−1 year−1 in the Kampar Peninsula8 may no longer be occurring. If we take the measured GHG balance of the intact forest site as a reference, and treat our data from the fourth Acacia rotation as representative of longer-term conditions, then the conversion of intact site to Acacia plantation results in a long-term net increase in GHG emissions of 18.1 ± 6.0 tCO2-eq ha−1 year−1 (Fig. 2).
Our study is the first, to our knowledge, to provide an estimate of CO2 emissions from tropical Acacia plantation on peat based on the eddy covariance method, over a full plantation rotation. The CO2 EF is critical to GHG inventories in Acacia plantations, given that, in Acacia plantation, about 90% of peat on-site GHG emissions is released as CO2 (Fig. 2). We were able to reduce the uncertainties associated with variations in flux during the full plantation rotation and in biomass removal at the end of the rotation, which make estimating the average C balance of high-latitude peatlands with multidecadal rotations highly problematic. Our directly measured CO2 balance of the Acacia plantation is half of the IPCC Tier 1 EF value of 73 tCO2 ha−1 year−1. Two of the key studies22,23 used to derive the Tier 1 EF were carried out in the same plantation area in the initial 3–8 years after drainage, so interregional differences cannot explain the discrepancy. Subsidence rates24,26,40 and CO2 emissions30,41,42 in tropical peatlands are reported to decrease over time following drainage. An increase in bulk density (0.08 g cm−3 during 3–8 years after drainage in refs. 22,23 versus 0.20 g cm−3 during 17–22 years after drainage in this study; Extended Data Table 1), owing to peat compaction during land preparation, may result in lower peat oxidative decomposition because of the increase in soil water content and decrease in soil gas diffusivity43. Furthermore, a decline in soil organic matter quality44 and nutrient availability over time may leave behind a more stable peat matrix, resulting in a decrease in substrate-driven rates of CO2 production41 from peat decomposition. Finally, not all of the emissions in the initial years after plantation establishment22,23 would come directly from peat C decomposition, given that considerable forest biomass residues would also contribute to the initial CO2 loss, with most42 of the forest residues decomposing in the initial years after conversion.
Some other factors may have contributed to lower than expected long-term plantation emissions in our study. Improved water management practices, reflected in higher average GWL than reported in previous studies22,23, may have reduced oxidation rates to some extent. We also measured a C input to the peat of around 12 tCO2 ha−1 year−1 (calculated as the difference between the oxidative peat decomposition (41.7 tCO2 ha−1 year−1; Extended Data Table 3) and the sum of NEE-CO2 (9.5 tCO2 ha−1 year−1) and C export in harvested wood (20.5 tCO2 ha−1 year−1)) over the full plantation rotation. This C input, derived from litter, roots, stumps and bark residues, is not measured during chamber and subsidence studies, which may have led to overestimation of net CO2 emissions. Although our results confirm that fibre wood plantations are substantial net GHG sources, these results indicate that there may be opportunities to increase soil C input through better post-harvest residue management. Further research is needed to confirm the potential scale of increase in C input that could realistically be achieved.
The data from our three study sites, along with four other published eddy covariance studies from tropical peatlands (Extended Data Table 6), conform well to a linear relationship between CO2 flux and GWL (R2 = 0.83, P < 0.05; Fig. 3), suggesting that measured emissions are broadly consistent with those of other studies that applied a similarly rigorous whole-ecosystem eddy covariance measurement approach. As is evident from Fig. 3, net CO2 fluxes and their relationships with GWL derived from eddy covariance studies are substantially lower than those obtained from chamber and subsidence studies in the same ecosystems. Although the number of published eddy covariance studies from tropical peatlands remains insufficient to establish whether these differences are systematic, a similar offset is evident in CO2 flux versus GWL relationships derived from eddy covariance data45 and chamber data46 for high-latitude peatlands. Although further data are needed, we therefore tentatively conclude that emissions from Acacia plantation are substantially lower than the current IPCC Tier 1 EFs, as a result of methodological limitations to the data available at the time of publication of the IPCC Wetlands Supplement20 and changes in peat physicochemical properties with time since drainage.
Our results should not be extrapolated to other agriculture on peat in the region (for example, sago, oil palm, rubber plantations etc.) or to other tropical peatlands, such as those of the Amazon and Congo basins, because they have different rainfall regimes, vegetation and peat-formation histories. Nevertheless, the strong linear relationship between CO2 flux and GWL shown in Fig. 3 does suggest that, when the average annual GWL is known, the peatland CO2 balance can be predicted with some degree of confidence. This is in line with work45,47 on high-latitude peatlands suggesting that GWLs are more important than local climate or other management factors. Furthermore, although the relationship between CO2 flux and GWL is steeper for tropical peatlands compared with the full set of high-latitude flux tower data collated in ref. 45, we found less difference than expected between the tropical data and the data presented from the UK and Irish sites in the same study (Fig. 3). This finding is in marked contrast to a recently published synthesis study suggesting that tropical peatlands are inherently more sensitive to CO2 loss following GWL drawdown48. However, given that ref. 48 incorporated the same chamber studies used to derive the IPCC Tier 1 EF, we believe that it may have overestimated rates of CO2 loss from tropical peatlands for the same reasons noted above.
Using our EFs, net GHG emissions from Acacia plantations on peat in Indonesia are calculated to be 20 Mt CO2-eq year−1 (based on the area of Acacia plantation on peat in Indonesia, 1.12 × 106 ha (ref. 21)). This equates to 1.1% of Indonesia’s most recently reported total GHG emissions in 2019 (ref. 49). Infrequent but intense fires are common in unmanaged degraded peatlands, particularly during prolonged drought driven by climate extreme events (for example, 2006, 2015 and 2019), and may result in higher GHG release to the atmosphere than peat decomposition49. GHG emissions at the degraded site are about 20% higher than those of the plantation site, indicating that establishment of Acacia plantation on previously degraded site could apparently result in lower long-term GHG emissions of −7.5 ± 6.5 tCO2-eq ha−1 year−1, as well as avoided emissions from bioenergy production (Fig. 2). Because the initial disturbance of this site occurred at a similar time to that at the plantation site (see Methods), it is probable that higher emissions from the degraded site are partly because of the decomposition of woody debris from fallen dead trees. Our results do not argue against full restoration of unmanaged degraded peatlands where this is achievable, as their ecosystem rehabilitation (that is, hydrological restoration and re-establishment of a closed forest canopy) offers an opportunity to restore and improve the ability of peatlands to sequester and retain C, but this will be critically dependent on protecting these areas from encroachment and fire.
Using our EFs from the intact and degraded sites, the results highlight that, despite evidence that they may now be losing C, avoided emissions from conserving all remaining intact peat swamp forests in Indonesia (2.0 × 106 ha) under Indonesia’s nationally determined contribution49 and emissions reduction from restoring 4.2 × 106 ha by 2050 under Indonesia’s Low Carbon scenario Compatible with the Paris Agreement target (LCCP)50 will avoid GHG emissions of around 160 MtCO2-eq year−1. This equates to around 40% of GHG emissions from peat decomposition in Indonesia in 2019 (ref. 49). This estimate is conservative. If some remaining intact peatlands are continuing to sequester CO2, the avoided emissions will be correspondingly higher. Our results clearly indicate that the net avoidance and reduction of GHG emissions resulting from peatland conservation, restoration and sustainable management represent a notable contribution to nationally determined contributions to a 1.5 °C world32.
Methods
Study area
This study was conducted in the Kampar Peninsula (Sumatra, Indonesia), an ombrogenous tropical peatland of around 700,000 ha that largely formed within the past 5,100 years (ref. 8). The base of the peatland is grey marine clays, over which peat varies from approximately 3 m deep near the river boundaries to over 11 m in the centre of the approximately 60-km-wide and more than 100-km-long peat dome (Fig. 1), with an average depth of 8 m. The peninsula experiences a humid tropical climate with the average monthly air temperature ranging from 26 to 29 °C (refs. 17,27). The variability in rainfall is influenced by monsoonal processes combined with El Niño–Southern Oscillation and IOD51,52. In general, the El Niño34 and positive IOD35 occur sequentially, with the positive IOD peaking a few months after the El Niño, exerting a strong combined effect on regional rainfall patterns26. The average annual rainfall for the past 8 years (2014–2021, with El Niño in 2015, La Niña in 2017 and a major positive IOD combined with an El Niño event in 2019) is 1,772 ± 201 mm. Rainfall varies seasonally, with two annual peaks, one in November–December and another in March–April. The land cover of the peninsula is characterized by a large central forest area that still has good-quality dense forest, representing one of the largest peat swamp forests in Southeast Asia. In some parts of the peninsula, selective logging took place in the 1990s, including the construction of access logging tracks and canals, especially around the periphery of the forest. However, some areas have never been logged and have been classified as intact peat swamp forest21. Most of the logged forest was converted to industrial fibre wood plantation and smallholder agriculture in the early 2000s. At present, the central forest area is surrounded by a mosaic of A. crassicarpa, oil palm plantation and degraded peat swamp forest with shrub and open land21 (Fig. 1).
At the experimental fibre wood plantation site, the peat swamp forest was disturbed by selective logging activity, including logging tracks and canals in the early 1990s. In the early 2000s, the area was converted to an Acacia plantation. This involved clearance of the remaining logged forest, artificial compaction during mechanical land preparation, installation of regularly spaced water management and access canals and planting of A. crassicarpa, which is harvested on a 4–5-year rotation. The area was not affected by fire before, during or after land-use change. Acacia crassicarpa (Leguminosae) is a fast-growing, N-fixing tree that is the principal fibre wood plantation species grown on peat soils in Southeast Asia. The typical plantation rotation period between planting of tree seedlings to harvest is 4–5 years, and a closed canopy develops in around 12 to 18 months. When measurements began in October 2016, the trees were already at the end of the third plantation rotation. All plantation compartments within a 2-km radius around the eddy covariance tower were harvested between October 2016 and April 2017. Tree height at harvest was in the range 19–24 m, determined from a vegetation survey in permanent sampling plots (20 m × 125 m) around the tower. Replanting for the fourth plantation rotation took place within two weeks after harvesting each individual compartment at a density of 1,667 trees per hectare (3 m × 2 m spacing). Five grams of chelated micronutrients per tree were applied around the seedlings during planting. All compartments within a 2-km radius of the eddy covariance tower were harvested between June and August 2021, when the average plantation age was 4.7 years, and replanting for the fifth plantation rotation took place within two weeks after harvesting. The ground surface in the plantation area is relatively even, without a hummock-hollow microtopography and with very little understory vegetation. The site soil characteristics are summarized in Extended Data Table 1. GWLs in the experimental plantation are actively managed by means of an extensive network of topographically defined water management zones, controlled by outlet sluices and supported by GWL monitoring. Water management zones consist of navigable canals, typically of 12 m width and 3 m depth, also used for transport25. Branch canals of 5–8 m width run perpendicular to these canals at a spacing of 500–800 m to form plantation compartments, which contain 1-m-deep field drains at a spacing of 75 m (ref. 25). An integrated climatologic footprint analysis53 indicated that (1) approximately 80% of measured fluxes derived from within 1,000 m in the upwind direction and thus originated within the Acacia plantation and (2) the water surface of ditches and canals represented 2% of the flux footprint (Fig. 1).
The second eddy covariance tower is located on the boundary of the degraded peatland and Acacia plantation (Fig. 1). To represent only the degraded peatland, half-hourly measurements in which the prevailing wind came from the plantation site (90° to 270°) were excluded, as is commonly done in eddy covariance studies54. The degraded site was selectively logged and drained in the late 1990s and early 2000s, whereas some parts were burned in 2014. The average canopy height was about 19 m. The tree density with a diameter at breast height of greater than 5 cm was 663 trees per hectare. Some large trees had been logged or fallen and many of those remaining were leaning. The site characteristics are summarized in Extended Data Table 1. The integrated climatologic footprint analysis53 indicated that approximately 80% of the fluxes were derived within 1,000 m in the upwind direction and the previously burnt area represented around 5% of the flux footprint (Fig. 1). The average eddy covariance footprint can be considered typical of many unmanaged degraded peatlands in Southeast Asia21.
The intact peat swamp forest structure is mixed with an uneven canopy (average canopy height = 32 m). The density of trees with a diameter at breast height greater than 5 cm was 1,343 stems per hectare. The vegetation and soil characteristics are summarized in Extended Data Table 1. The GWL fluctuates following the rainfall variation because of the ombrotrophic nature of the area17,27. An integrated climatologic footprint analysis53 indicated that approximately 80% of the fluxes were derived within 1,000 m in the upwind direction (Fig. 1) and thus originated from intact forest with neither logging nor canal-construction activity21. Some long-term regional effects of hydrological management of surrounding plantations cannot be ruled out, but a previous analysis suggested that the strongest effects occurred within 300 m of the plantation boundary25, and recent multivariate analysis indicates that subsidence in the interior forest is independent of distance from plantation canals26. The nearest active plantation is 3.5 km from the flux tower and well outside the flux footprint. Further, to avoid any possible boundary effect and associated bias, measurements from a wind direction between 78° and 191° were excluded in this study (Fig. 1).
Eddy covariance provides half-hourly measurements of turbulent exchanges between an entire ecosystem and the atmosphere above the vegetation canopy54. Hence, eddy covariance measurements incorporate all existing sources and uptakes that can vary substantially within an ecosystem in both space and time arising from variation in environmental conditions. Given the flat terrain (slope less than 0.05%), using the measured vegetation-canopy height and wind speed, the estimated 80% eddy covariance flux footprints represent an area of interest of around 1,000 m radius (Fig. 1). Flux measurements with the eddy covariance technique are expensive and high maintenance, and few studies include replicated measurements from several towers in tropical forested ecosystems. The relatively close proximity of the Acacia plantation and intact and degraded sites (Fig. 1) within the same peat landscape avoids potentially confounding variables such as differences in past natural succession55 and peat formation8.
Eddy covariance and environmental variable measurements
Each eddy covariance system consisted of an enclosed-path CO2/H2O analyser (LI-7200, LI-COR) to measure CO2 and H2O concentrations, an open-path CH4 analyser (LI-7700, LI-COR) to measure CH4 concentrations and a three-dimensional sonic anemometer (WindMaster Pro 3-Axis Anemometer, Gill Instruments) to measure the orthogonal components of wind-speed fluctuations. Eddy covariance sensors were mounted at the top of each tower to ensure complete exposure in all directions (Fig. 1). The filters of the CO2 analyser were manually cleaned, either biweekly or if the flow drive increased above 80% (indicating filter clogging). The mirrors of the CH4 analyser were cleaned automatically either at 05:00 local time every day or if the received-signal-strength indicator dropped below 20%, because CH4 data become noisy below this threshold. Furthermore, the upper and lower mirrors of the CH4 analyser were manually cleaned on a biweekly basis. The raw eddy covariance turbulence data were recorded at 10 Hz using an analyser interface unit (LI-7550, LI-COR) and were stored on a removable flash disk (Industrial Grade USB Flash Disk, APRO).
A quantum sensor (LI-190SL-50, LI-COR) was mounted at the top of each tower to measure incoming photosynthetic photon flux density (PPFD). A radiometer (CNR4, Kipp & Zonen) was also mounted at the top of each tower to measure global and net radiation. Vertical profiles of relative humidity and air temperature were measured using air-temperature and humidity probes (HMP155, Vaisala), which were installed inside ventilated radiation shields at five heights from the ground surface, 3, 7, 13, 23 and 40 m for the plantation site, 3, 7, 14, 21 and 40 m for the degraded site and 4, 11, 20, 29 and 48 m for the intact site. Vertical profiles of the CO2 concentrations were measured by air sampling at four heights, 3, 12, 22 and 40 m for the plantation site, 3, 14, 21 and 40 m for the degraded site and, 4, 11, 29 and 48 m for the intact site, to calculate the flux-storage56 term below the measurement height using a closed-path CO2 analyser (LI-8100, LI-COR). The air-sampling intakes were automatically changed every 90 s and the CO2 concentration was measured for the last 10 s of every 90-s sampling time at each sampling height and recorded using a data logger (LI-8100, LI-COR); therefore, one rotation of measurements took 6 min in every 30 min. Both the enclosed-path and closed-path CO2 analysers were calibrated every three months using reference gases with concentrations of 396 and 444 ppm CO2 in air (certified grade ±1 ppm) and ultrahigh-purity nitrogen as the zero-point gas. The soil temperature was measured at 0.15 m below the hollow peat surface using a temperature probe (HydraProbe II, Stevens Water Monitoring Systems) from September 2017 until November 2018 and from October 2016 until June 2020 with three replicates at the intact and plantation sites, respectively. From November 2019 until May 2022, the soil temperatures were measured at the intact site and from November 2019 until May 2021 at the plantation site with two replicates using a temperature probe (AquaCheck, South Africa). The soil temperatures were not measured at the degraded site owing to site logistic issues.
All meteorological sensors took measurements every second and were recorded as 1-min averages using a data logger (Model 9210 XLite, Sutron). Each measuring system was powered using five solar panels (65-W solar panel, SunWize), along with eight rechargeable batteries (6 V and 305 Ah, Sun Xtender). The daily rainfall (mm day−1) was manually measured using three, two and three bucket-rain gauges within a distance of 11 km from the tower location at the plantation, degraded and intact sites, respectively. Each rain gauge was installed 1.5 m above the ground, in an open area so that rainfall was not affected by surrounding vegetation.
The GWL was monitored as the water elevation relative to the ground surface, taking the base of the hollows as a datum17. Data were recorded as negative distance below the surface, with positive values indicating ponding above the surface. The GWL loggers (four around the plantation tower, one in the degraded site and six in the intact site) to record the GWL every 30 min using a pressure transducer (Levelogger Model 3001, Solinst) were placed in perforated polyvinyl chloride pipes that were inserted vertically into the peat and anchored into the underlying clay (Fig. 1). Each GWL logger also recorded the water temperature in the pipe 1.5 m below the peat surface. Further GWL data were manually recorded biweekly at seven and three locations at the plantation and degraded sites, respectively, and on a quarterly basis at eight more locations in the intact site (Fig. 1).
Peat subsidence was measured at 11, four and 14 locations in the plantation, degraded and intact sites, respectively (Fig. 1), with hollow, perforated 5-cm-diameter polyvinyl chloride poles inserted vertically into the peat and anchored into the underlying mineral subsoil following the approach described in ref. 25. Annual average subsidence rates were derived from measurements during October 2016–May 2021 for the plantation site and during December 2017–May 2022 for the degraded and intact sites.
For peat physical and chemical properties of the surface layer (0–50 cm), four plots in each of the Acacia plantation and degraded sites and three plots in the intact site were randomly selected within the eddy covariance flux footprint (200–1,000 m distance from each tower location; Fig. 1). At each plot, ten subsamples within a 200-m radius were composited. Peat samples for bulk density, pH and ash content were collected in September 2017, February 2019 and September 2019 in the intact site, June 2017, January 2018, October 2018 and February 2019 in the degraded site and June 2017, February 2018, October 2018, February 2019 and October 2019 in the Acacia plantation. Samples for soil C, N, nitrate and ammonium content were collected in August 2020 and October 2021 for all sites.
For the Acacia plantation site, time‐integrated NEE-CO2 over the plantation rotation was combined with C export in the harvested wood. Total C export in harvested wood and delivered to the mill from the total footprint area of 220 ha over the average plantation age of 4.7 years was calculated using a basic density of 455 ± 25 kg m−3 and average C content of 48.2% (refs. 56,57,58,59). The exported wood is converted into pulp products and biomass fuel for bioenergy generation. We applied the conservative assumption that all C in exported wood would be returned to the atmosphere as CO2. Intact and degraded sites were considered to have had no biomass C export during the study period.
The biomass C loss owing to land-use change was calculated from aboveground and belowground biomass C-stock differences between the intact site and the Acacia plantation and degraded areas. Aboveground and belowground biomass were determined using seven permanent sampling plots (20 m × 125 m) at each site and following the allometric equations described in refs. 60,61 for the intact and degraded sites and ref. 62 for the Acacia plantation. A time horizon of 100 years of land being used after conversion is chosen on the basis of ISO 14067 on C footprint.
Eddy covariance data processing
The eddy covariance fluxes of CO2, CH4 and evapotranspiration were computed from the 10-Hz concentration and vertical wind velocity data using EddyPro software (version 6.2.0, LI-COR) at a standard half-hour averaging interval54. A despiking procedure was applied to detect and eliminate individual out-of-range values for vertical wind velocity and concentration data63. Detrending was carried out using the block-averaging method. A coordinate correction was applied to force the average vertical wind velocity to zero by the planar-fit method64. Frequency response loss corrections were applied to compensate for the flux losses at low and high frequencies65. The Webb–Pearman–Leuning correction66 for air-density fluctuations induced by temperature (thermal expansion) and water vapour (dilution) was applied.
Differences between deployment-specific variables, that is, the sensor separation distance and instrument placement, were considered when processing the data. The half-hourly CO2 storage below the flux measurement height was calculated from the four-point vertical profiles of CO2 concentration, relative humidity and air temperature by temporal interpolation56. Finally, the net ecosystem CO2 exchange was calculated as the sum of the storage flux and the eddy covariance flux. Owing to the large power requirement and cost of a separate CH4 analyser, we could not conduct CH4-profile measurements to calculate the CH4 storage67. In theory, accumulated CH4 below the canopy during the nighttime is probably released and measured by the eddy covariance system following the onset of turbulence after sunrise, and the bias on annual sums should be negligible67.
After a set of quality controls68,69,70 and system malfunctions and power-supply failure mainly because of lightning strikes, the numbers of high-quality measurements during the course of the study were 37%, 34% and 34% for CO2, 26%, 29% and 25% for CH4 and 34%, 34% and 28% for evapotranspiration in the plantation, degraded and intact sites, respectively. A similar range of 25–50% has been reported for other eddy covariance studies in tropical forested peatlands42,56. The remaining half-hourly measurements that met all the quality criteria totalled 30,196, 14,330 and 18,136 for CO2, 21,305, 12,721 and 13,026 for CH4 and 27,965, 14,437 and 14,919 for evapotranspiration for the plantation, degraded (270–90° wind direction) and intact (191–78° wind direction) sites, respectively.
We gap-filled both low-quality and missing data, as is commonly done in eddy covariance studies17,27,42,56,71,72,73,74,75. Following ref. 17, we applied three gap-filling approaches for CO2: (1) marginal distribution sampling (MDS)56,76, (2) artificial neural network (ANN)73 and (3) random forest (RF)74 separately for the daytime (06:00–18:00 local time) and the nighttime (18:00–06:00 local time) data. To avoid any possible gap-filling bias in estimates of CO2, CH4 and evapotranspiration, we used the average of the three approaches17. We applied principal component analysis as an input to the algorithms to address multidriver dependency of CO2 exchange and reduce the internal complexity of the algorithmic structures for the MDS approach77, using PPFD, vapour-pressure deficit (VPD) and air temperature during daytime. Nighttime CO2 exchanges were considered equivalent to the ecosystem respiration (Reco) value78. The GWL is reported as the main controlling factor of Reco from tropical peatlands17,56. Therefore, we used the GWL, air temperature and soil temperature as environmental factors for the lookup table to derive the nighttime CO2 exchanges using the MDS gap-filling algorithm. Following other regional eddy covariance studies in peat swamp forests17,56, we performed MDS gap-filling using the REddyProc package (https://CRAN.R-project.org/package=REddyProc) on a half-hourly basis77. ANN and RF procedures were iterated 20 times. For ANN and RF, PPFD, VPD, air temperature, GWL and friction velocity were used as predictive variables for the daytime and the PPFD and VPD data were excluded in the nighttime.
We applied the above gap-filling approaches for CH4 and evapotranspiration as well. For CH4, we used GWL, VPD, air temperature, friction velocity, latent heat flux, sensible heat flux, atmospheric pressure and global radiation in the daytime and latent heat flux and global radiation were excluded in the nighttime for ANN and RF. For MDS, we used latent heat flux, GWL and air temperature during the daytime and GWL, air temperature and soil temperature during the nighttime. For evapotranspiration, we applied net radiation instead of PPFD during the daytime, whereas net radiation and VPD were excluded during the nighttime. After gap filling, we corrected the daily evapotranspiration for the energy imbalance using net radiation, sensible heat and latent heat as described in ref. 79.
The flux random uncertainty was calculated following ref. 80. The standard deviation of three different flux values derived from friction velocity thresholds of the 5th, 50th and 95th percentiles were applied as an uncertainty because of the friction velocity threshold using the REddyProc package77. The gap-filling flux uncertainty was calculated from the standard deviation of the MDS procedure77. Averages of the 20 ANN and RF modelled values were used to fill gaps and the standard deviation was used to quantify the uncertainty owing to gap filling. The total uncertainty in eddy covariance measurements of CO2, CH4 and evapotranspiration included gap-filling, random and friction velocity uncertainty81. The annual estimate of CO2, CH4, evapotranspiration and GHG balance includes total uncertainty calculated using the propagation of errors law.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Data availability
All data that support the findings of this study are archived on Zenodo at https://doi.org/10.5281/zenodo.7728463.
References
Lähteenoja, O. et al. The large Amazonian peatland carbon sink in the subsiding Pastaza-Marañón foreland basin, Peru. Glob. Change Biol. 18, 164–178 (2012).
Dargie, G. C. et al. Age, extent and carbon storage of the central Congo Basin peatland complex. Nature 542, 86–90 (2017).
Warren, M., Hergoualc’h, K., Kauffman, J. B., Murdiyarso, D. & Kolka, R. An appraisal of Indonesia’s immense peat carbon stock using national peatland maps: uncertainties and potential losses from conversion. Carbon Balance Manag. 12, 12 (2017).
Gumbricht, T. et al. An expert system model for mapping tropical wetlands and peatlands reveals South America as the largest contributor. Glob. Change Biol. 36, 335 (2017).
Kurnianto, S. et al. Carbon accumulation of tropical peatlands over millennia: a modeling approach. Glob. Change Biol. 21, 431–444 (2015).
Dommain, R., Couwenberg, J. & Joosten, H. Development and carbon sequestration of tropical peat domes in south-east Asia: links to post-glacial sea-level changes and Holocene climate variability. Quat. Sci. Rev. 30, 999–1010 (2011).
Cobb, A. R. et al. How temporal patterns in rainfall determine the geomorphology and carbon fluxes of tropical peatlands. Proc. Natl Acad. Sci. USA 114, E5187–E5196 (2017).
Hapsari, K. A., Jennerjahn, T., Nugroho, S. H., Yulianto, E. & Behling, H. Sea level rise and climate change acting as interactive stressors on development and dynamics of tropical peatlands in coastal Sumatra and South Borneo since the Last Glacial Maximum. Glob. Change Biol. 28, 3459–3479 (2022).
Gallego-Sala, A. V. et al. Latitudinal limits to the predicted increase of the peatland carbon sink with warming. Nat. Clim. Change 8, 907–913 (2018).
Loisel, J. et al. Expert assessment of future vulnerability of the global peatland carbon sink. Nat. Clim. Change 11, 70–77 (2021).
Wang, S., Zhuang, Q., Lähteenoja, O., Draper, F. C. & Cadillo-Quiroz, H. Potential shift from a carbon sink to a source in Amazonian peatlands under a changing climate. Proc. Natl Acad. Sci. 115, 12407–12412 (2018).
Li, W. et al. Future precipitation changes and their implications for tropical peatlands. Geophys. Res. Lett. 34, L01403 (2007).
Dadap, N. C. et al. Climate change-induced peatland drying in Southeast Asia. Environ. Res. Lett. 17, 074026 (2022).
Dommain, R., Couwenberg, J., Glaser, P. H., Joosten, H. & Suryadiputra, I. N. N. Carbon storage and release in Indonesian peatlands since the last deglaciation. Quat. Sci. Rev. 97, 1–32 (2014).
Garcin, Y. et al. Hydroclimatic vulnerability of peat carbon in the central Congo Basin. Nature 612, 277–282 (2022).
Swails, E. et al. The response of soil respiration to climatic drivers in undrained forest and drained oil palm plantations in an Indonesian peatland. Biogeochemistry 142, 37–51 (2019).
Deshmukh, C. S. et al. Conservation slows down emission increase from a tropical peatland in Indonesia. Nat. Geosci. 14, 484–490 (2021).
Leifeld, J. & Menichetti, L. The underappreciated potential of peatlands in global climate change mitigation strategies. Nat. Commun. 9, 1071 (2018).
Austin, K. G. et al. A review of land-based greenhouse gas flux estimates in Indonesia. Environ. Res. Lett. 13, 055003 (2018).
Drösler, M. et al. in 2013 Supplement to the 2006 IPCC Guidelines for National Greenhouse Gas Inventories: Wetlands (eds Hiraishi, T. et al.) 2.1–2.79 (IPCC, 2013).
Miettinen, J., Shi, C. & Liew, S. C. Land cover distribution in the peatlands of Peninsular Malaysia, Sumatra and Borneo in 2015 with changes since 1990. Glob. Ecol. Conserv. 6, 67–78 (2016).
Hooijer, A. et al. Subsidence and carbon loss in drained tropical peatlands. Biogeosciences 9, 1053–1071 (2012).
Jauhiainen, J., Hooijer, A. & Page, S. E. Carbon dioxide emissions from an Acacia plantation on peatland in Sumatra, Indonesia. Biogeosciences 9, 617–630 (2012).
Hoyt, A. M., Chaussard, E., Seppalainen, S. S. & Harvey, C. F. Widespread subsidence and carbon emissions across Southeast Asian peatlands. Nat. Geosci. 13, 435–440 (2020).
Evans, C. D. et al. Rates and spatial variability of peat subsidence in Acacia plantation and forest landscapes in Sumatra, Indonesia. Geoderma 338, 410–421 (2019).
Evans, C. D. Long-term trajectory and temporal dynamics of tropical peat subsidence in relation to plantation management and climate. Geoderma 428, 116100 (2022).
Deshmukh, C. S. et al. Impact of forest plantation on methane emissions from tropical peatland. Glob. Change Biol. 26, 2477–2495 (2020).
Wong, G. X. et al. How do land use practices affect methane emissions from tropical peat ecosystems? Agric. For. Meteorol. 107869, 282–283 (2020).
Swails, E., Hergoualc’h, K., Verchot, L., Novita, N. & Lawrence, D. Spatio-temporal variability of peat CH4 and N2O fluxes and their contribution to peat GHG budgets in Indonesian forests and oil palm plantations. Front. Environ. Sci. 9, 617828 (2021).
Swails, E., Hergoualc’h, K., Deng, J., Frolking, S. & Novita, N. How can process-based modeling improve peat CO2 and N2O emission factors for oil palm plantations?. Sci. Total Environ. 839, 156153 (2022).
Prairie, Y. T. et al. Greenhouse gas emissions from freshwater reservoirs: what does the atmosphere see? Ecosystems 21, 1058–1071 (2018).
Roe, S. et al. Contribution of the land sector to a 1.5 °C world. Nat. Clim. Change 9, 817–828 (2019).
Ruwaimana, M., Anshari, G. Z., Silva, L. C. R. & Gavin, D. G. The oldest extant tropical peatland in the world: a major carbon reservoir for at least 47 000 years. Environ. Res. Lett. 15, 11 (2020).
Cai, W. et al. Increasing frequency of extreme El Niño events due to greenhouse warming. Nat. Clim. Change 4, 111–116 (2014).
Cai, W. et al. Increased frequency of extreme Indian Ocean Dipole events due to greenhouse warming. Nature 510, 254–258 (2014).
Yupi, H. M., Inoue, T., Bathgate, J. & Putra, R. Concentrations, loads and yields of organic carbon from two tropical peat swamp forest streams in Riau Province, Sumatra, Indonesia. Mires Peat 18, 1–15 (2016).
Cook, S. et al. Fluvial organic carbon fluxes from oil palm plantations on tropical peatland. Biogeosciences 15, 7435–7450 (2018).
Evans, C. D., Renou-Wilson, F. & Strack, M. The role of waterborne carbon in the greenhouse gas balance of drained and re-wetted peatlands. Aquat. Sci. 78, 573–590 (2016).
Neubauer, S. C. & Megonigal, J. P. Moving beyond global warming potentials to quantify the climatic role of ecosystems. Ecosystems 18, 1000–1013 (2015).
Umarhadi, D. A. et al. Tropical peat subsidence rates are related to decadal LULC changes: insights from InSAR analysis. Sci. Total Environ. 816, 151561 (2022).
Swails, E. et al. Will CO2 emissions from drained tropical peatlands decline over time? Links between soil organic matter quality, nutrients, and C mineralization rates. Ecosystems 21, 868–885 (2018).
McCalmont, J. et al. Short- and long-term carbon emissions from oil palm plantations converted from logged tropical peat swamp forest. Glob. Change Biol. 27, 2361–2376 (2021).
Melling, L., Goh, K. J., Chaddy, A. & Hatano, R. Soil CO2 fluxes from different ages of oil palm in tropical peatland of Sarawak, Malaysia as influenced by environmental and soil properties. Acta Hortic. 982, 25–35 (2013).
Hoyos-Santillan, J. et al. Quality not quantity: organic matter composition controls of CO2 and CH4 fluxes in neotropical peat profiles. Soil Biol. Biochem. 103, 86–96 (2016).
Evans, C. D. et al. Overriding water table control on managed peatland greenhouse gas emissions. Nature 593, 548–552 (2021).
Tiemeyer, B. et al. A new methodology for organic soils in national greenhouse gas inventories: data synthesis, derivation and application. Ecol. Indic. 109, 105838 (2020).
Ma, L. et al. A globally robust relationship between water table decline, subsidence rate, and carbon release from peatlands. Commun Earth Environ 3, 254 (2022).
Zou, J. et al. Rewetting global wetlands effectively reduces major greenhouse gas emissions. Nat. Geosci. 15, 627–632 (2022).
Ministry of Environment and Forestry. Indonesia. Third Biennial Update Report. Under the United Nations Framework Convention on Climate Change (2021).
Government of Indonesia. Indonesia. Long-Term Strategy for Low Carbon and Climate Resilience 2050 (2021).
Saji, N. H., Goswami, B. N., Vinayachandran, P. N. & Yamagata, T. A dipole mode in the tropical Indian Ocean. Nature 401, 360–363 (1999).
Alsepan, G. & Minobe, S. Relations between interannual variability of regional-scale Indonesian precipitation and large-scale climate modes during 1960–2007. J. Climate 33, 5271–5291 (2020).
Kljun, N., Calanca, P., Rotach, M. W. & Schmid, H. P. A simple two-dimensional parameterisation for Flux Footprint Prediction (FFP). Geosci. Model Dev. 8, 3695–3713 (2015).
Aubinet, M. et al. Estimates of the annual net carbon and water exchange of forests: the EUROFLUX methodology. Adv. Ecol. Res. 30, 113–175 (2000).
Cole, L. E. S., Bhagwat, S. A. & Willis, K. J. Long-term disturbance dynamics and resilience of tropical peat swamp forests. J. Ecol. 103, 16–30 (2015).
Hirano, T. et al. Effects of disturbances on the carbon balance of tropical peat swamp forests. Glob. Change Biol. 18, 3410–3422 (2012).
Aalde, H. et al. in 2006 IPCC Guidelines for National Greenhouse Gas Inventories 4.1–4.83 (IPCC, 2006).
He, B. et al. Carbon storage and distribution in Acacia crassicarpa plantation ecosystem [in Chinese]. J. Nanjing For. Univ. Nat. Sci. 33, 46–50 (2009).
Zhang, H., Jiang, Y., Song, M., He, J. & Guan, D. Improving understanding of carbon stock characteristics of Eucalyptus and Acacia trees in southern China through litter layer and woody debris. Sci. Rep. 10, 4735 (2020). (2020).
Mokany, K., Raison, R. & Prokushkin, A. S. Critical analysis of root:shoot ratios in terrestrial biomes. Glob. Change Biol. 12, 84–96 (2006).
Manuri, S. et al. Tree biomass equations for tropical peat swamp forest ecosystems in Indonesia. For. Ecol. Manag. 334, 241–253 (2014).
Setiawan, B. I., Siregar, S. T., Nawari, Nugroho, A. & Sharma, M. in 15th International PEAT Congress (IPC 2016) 514–517 (International Peatland Society, 2016).
Vickers, D. & Mahrt, L. Quality control and flux sampling problems for tower and aircraft data. J. Atmos. Ocean. Technol. 14, 512–526 (1997).
Wilczak, J. M., Oncley, S. P. & Stage, S. A. Sonic anemometer tilt correction algorithms. Bound.-Layer Meteorol. 99, 127–150 (2001).
Massman, W. J. A simple method for estimating frequency response corrections for eddy covariance systems. Agric. For. Meteorol. 104, 185–198 (2000).
Webb, E. K., Pearman, G. I. & Leuning, R. Correction of flux measurements for density effects due to heat and water vapor transfer. Q. J. R. Meteorol. Soc. 106, 85–100 (1980).
Xu, K. E. et al. The eddy-covariance storage term in air: consistent community resources improve flux measurement reliability. Agric. For. Meteorol. 279, 107734 (2019).
Foken, T. & Wichura, B. Tools for quality assessment of surface-based flux measurements. Agric. For. Meteorol. 78, 83–105 (1996).
Mauder, M. et al. A strategy for quality and uncertainty assessment of long-term eddy-covariance measurements. Agric. For. Meteorol. 169, 122–135 (2013).
Papale, D. et al. Towards a standardized processing of net ecosystem exchange measured with eddy covariance technique: algorithms and uncertainty estimation. Biogeosciences 3, 571–583 (2006).
Falge, E. et al. Gap-filling strategies for defensible annual sums of net ecosystem exchange. Agric. For. Meteorol. 107, 43–69 (2001).
Moffat, A. M. et al. Comprehensive comparison of gap‐filling techniques for eddy covariance net carbon fluxes. Agric. For. Meteorol. 147, 209–232 (2007).
Papale, D. & Valentini, R. A new assessment of European forests carbon exchanges by eddy fluxes and artificial neural network spatialization. Glob. Change Biol. 9, 525–535 (2003).
Xu, T. et al. Evaluating different machine learning methods for upscaling evapotranspiration from flux towers to the regional scale. J. Geophys. Res. Atmos. 123, 8674–8690 (2018).
Kim, Y. et al. Gap‐filling approaches for eddy covariance methane fluxes: a comparison of three machine learning algorithms and a traditional method with principal component analysis. Glob. Change Biol. 26, 1499–1518 (2020).
Kiew, F. et al. CO2 balance of a secondary tropical peat swamp forest in Sarawak, Malaysia. Agric. For. Meteorol. 248, 494–501 (2018).
Wutzler, T. et al. Basic and extensible post-processing of eddy covariance flux data with REddyProc. Biogeosciences 15, 5015–5030 (2018).
Reichstein, M. et al. On the separation of net ecosystem exchange into assimilation and ecosystem respiration: review and improved algorithm. Glob. Change Biol. 11, 1424–1439 (2005).
Hirano, T., Kusin, K., Limin, S. & Osaki, M. Evapotranspiration of tropical peat swamp forests. Glob. Change Biol. 21, 1914–1927 (2015).
Finkelstein, P. L. & Sims, P. F. Sampling error in eddy correlation flux measurements. J. Geophys. Res. 106, 3503–3509 (2001).
Deventer, M. J. et al. Error characterization of methane fluxes and budgets derived from a long-term comparison of open- and closed-path eddy covariance systems. Agric. For. Meteorol. 278, 107638 (2019).
Griffis, T. J. et al. Hydrometeorological sensitivities of net ecosystem carbon dioxide and methane exchange of an Amazonian palm swamp peatland. Agric. For. Meteorol. 295, 108167 (2020).
Wong, G. X. et al. How do land use practices affect methane emissions from tropical peat ecosystems? Agric. For. Meteorol. 282–283, 107869 (2020).
Azizan, S. N. F. et al. Comparing GHG emissions from drained oil palm and recovering tropical peatland forests in Malaysia. Water 13, 3372 (2021).
Melling, L., Hatano, R. & Goh, K. J. Nitrous oxide emissions from three ecosystems in tropical peatland of Sarawak, Malaysia. Soil Sci. Plant Nutr. 53, 792–805 (2007).
Jauhiainen, J. et al. Nitrous oxide fluxes from tropical peat with different disturbance history and management. Biogeosciences 9, 1337–1350 (2012).
Inubushi, K., Furukawa, Y., Hadi, A., Purnomo, E. & Tsuruta, H. Seasonal changes of CO2, CH4 and N2O fluxes in relation to land-use change in tropical peatlands located in coastal area of South Kalimantan. Chemosphere 52, 603–608 (2003).
Hergoualc’h, K. et al. Spatial and temporal variability of soil N2O and CH4 fluxes along a degradation gradient in a palm swamp peat forest in the Peruvian Amazon. Glob. Change Biol. 26, 7198–7216 (2020).
Teh, Y. A., Murphy, W. A., Berrio, J., Boom, A. & Page, S. E. Seasonal variability in methane and nitrous oxide fluxes from tropical peatlands in the western Amazon basin. Biogeosciences 14, 3669–3683 (2017).
Adji, F. F., Hamada, Y., Darang, U., Limin, S. H. & Hatan, R. Effect of plant-mediated oxygen supply and drainage on greenhouse gas emission from a tropical peatland in Central Kalimantan, Indonesia. Soil Sci. Plant Nutr. 60, 216–230 (2014).
Acknowledgements
The establishment and operation of the eddy covariance towers and associated data collection were financed by Asia Pacific Resources International Ltd. (APRIL) and Riau Ecosystem Restoration (RER).
Author information
Authors and Affiliations
Contributions
C.S.D. conceived the study. C.S.D., A.P.S. and A.R.D. completed eddy covariance data processing. Nardi, A.P.S., Nurholis, M.H., C.S.D., A.R., R.E.M., S.K. and Y.S. collected data and maintained and calibrated the eddy covariance instruments. C.S.D. conceived the paper and wrote the initial draft, to which all authors provided critical contributions and approved submission.
Corresponding author
Ethics declarations
Competing interests
C.D.E., S.E.P., S.S., F.A. and D.A. contributed to this paper as part of their role in the Independent Peat Expert Working Group (IPEWG), which was set up by Asia Pacific Resources International Ltd. (APRIL) to provide objective science-based advice on peatland management. The contribution of A.R.D. was also supported by APRIL to provide technical guidance on the eddy covariance data processing, including quality controls and gap-filling protocols. C.S.D., Nardi, A.P.S., Nurholis, M.H., A.R., R.E.M., S.K. and Y.S. are employed by APRIL to conduct field measurements, including eddy covariance instruments maintenance and calibration. The funders had no role in the interpretation of data, in the writing of the manuscript or in the decision to publish the results. The authors declare that all views expressed are their own.
Peer review
Peer review information
Nature thanks Daniel Murdiyarso and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Acacia plantation, degraded and intact peat swamp forest in Sumatra, Indonesia, are emitting CO2 and CH4 to the atmosphere.
Cumulative measured net CO2 (a) and net CH4 (b) fluxes with cumulative flux uncertainty (random error, friction velocity threshold and gap-filling approach) at the Acacia plantation (blue), degraded site (red) and intact site (green). Carbon export in harvested wood at the Acacia plantation is added in the end of plantation rotation, conservatively assuming that all harvested C would be returned to the atmosphere as CO2. Intact and degraded sites were considered to have had no biomass C export during the study period. Positive values indicate emission to the atmosphere.
Extended Data Fig. 2 GWL is controlled by the balance between rainfall and evapotranspiration at intact peat swamp forest in Sumatra, Indonesia.
a, Time series of average GWL from three piezometers spanning 12 km with difference between 90 days moving average of rainfall and evapotranspiration. Negative difference indicates rainfall deficit. Positive and negative GWL values indicate the water level above and below the peat hollow surface, respectively. Diel pattern of evapotranspiration during dry season (February and July) (b) and wet season (April and November) (c) over the measurement periods show negligible evapotranspiration in the nighttime. The boxes show the median value and the interquartile range and whiskers denote the full range.
Extended Data Fig. 3 Soil N2O emissions increase as GWLs decrease in tropical peatlands.
Temporal variation (a) and spatial variation (b) in soil N2O fluxes from Acacia plantation (blue), degraded site (red) and intact site (green). The boxes show the median value and the interquartile range and whiskers denote the full range of all chambers. The plus signs (+) in the boxes of panel b show the average values. The n values represent the total number of soil N2O flux measurements. c, Relationship between N2O fluxes and GWL were derived from soil flux chamber measurements on various land uses across tropical peatlands. Positive and negative GWL values indicate the water level above and below the peat surface, respectively. Positive flux value indicates emission to the atmosphere and negative value indicates uptake by the soil.
Supplementary information
Supplementary Information
Supplementary Methods 1–4 and Supplementary References.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Deshmukh, C.S., Susanto, A.P., Nardi, N. et al. Net greenhouse gas balance of fibre wood plantation on peat in Indonesia. Nature 616, 740–746 (2023). https://doi.org/10.1038/s41586-023-05860-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-05860-9
This article is cited by
-
Large variation in carbon dioxide emissions from tropical peat swamp forests due to disturbances
Communications Earth & Environment (2024)
-
Global observation gaps of peatland greenhouse gas balances: needs and obstacles
Biogeochemistry (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.