Abstract
Gastrointestinal (GI) discomfort is a hallmark of most gut disorders and represents an important component of chronic visceral pain1. For the growing population afflicted by irritable bowel syndrome, GI hypersensitivity and pain persist long after tissue injury has resolved2. Irritable bowel syndrome also exhibits a strong sex bias, afflicting women three times more than men1. Here, we focus on enterochromaffin (EC) cells, which are rare excitable, serotonergic neuroendocrine cells in the gut epithelium3,4,5. EC cells detect and transduce noxious stimuli to nearby mucosal nerve endings3,6 but involvement of this signalling pathway in visceral pain and attendant sex differences has not been assessed. By enhancing or suppressing EC cell function in vivo, we show that these cells are sufficient to elicit hypersensitivity to gut distension and necessary for the sensitizing actions of isovalerate, a bacterial short-chain fatty acid associated with GI inflammation7,8. Remarkably, prolonged EC cell activation produced persistent visceral hypersensitivity, even in the absence of an instigating inflammatory episode. Furthermore, perturbing EC cell activity promoted anxiety-like behaviours which normalized after blockade of serotonergic signalling. Sex differences were noted across a range of paradigms, indicating that the EC cell–mucosal afferent circuit is tonically engaged in females. Our findings validate a critical role for EC cell–mucosal afferent signalling in acute and persistent GI pain, in addition to highlighting genetic models for studying visceral hypersensitivity and the sex bias of gut pain.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information).
References
Enck, P. et al. Irritable bowel syndrome. Nat. Rev. Dis. Primers 2, 16014 (2016).
Grundy, L., Erickson, A. & Brierley, S. M. Visceral pain. Annu. Rev. Physiol. 81, 261–284 (2019).
Bellono, N. W. et al. Enterochromaffin cells are gut chemosensors that couple to sensory neural pathways. Cell 170, 185–198 (2017).
Racke, K. & Schworer, H. Characterization of the role of calcium and sodium channels in the stimulus secretion coupling of 5-hydroxytryptamine release from porcine enterochromaffin cells. Naunyn Schmiedebergs Arch. Pharmacol. 347, 1–8 (1993).
Strege, P. R. et al. Sodium channel NaV1.3 is important for enterochromaffin cell excitability and serotonin release. Sci. Rep. 7, 15650 (2017).
Gershon, M. D. Serotonin is a sword and a shield of the bowel: serotonin plays offense and defense. Trans. Am. Clin. Climatol. Assoc. 123, 268–280 (2012).
Koh, A., De Vadder, F., Kovatcheva-Datchary, P. & Bäckhed, F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell 165, 1332–1345 (2016).
Farup, P. G., Rudi, K. & Hestad, K. Faecal short-chain fatty acids—a diagnostic biomarker for irritable bowel syndrome? BMC Gastroenterol. 16, 51 (2016).
Gribble, F. M. & Reimann, F. Enteroendocrine cells: chemosensors in the intestinal epithelium. Annu. Rev. Physiol. 78, 277–299 (2016).
Gribble, F. M. & Reimann, F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat. Rev. Endocrinol. 15, 226–237 (2019).
Liddle, R. A. Neuropods. Cell Mol. Gastroenterol. Hepatol. 7, 739–747 (2019).
Kaelberer, M. M. et al. A gut–brain neural circuit for nutrient sensory transduction. Science 361, eaat5236 (2018).
Treichel, A. J. et al. Specialized mechanosensory epithelial cells in mouse gut intrinsic tactile sensitivity. Gastroenterology 162, 535–547 (2022).
Nozawa, K. et al. TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc. Natl Acad. Sci. USA 106, 3408–3413 (2009).
Mawe, G. M. & Hoffman, J. M. Serotonin signalling in the gut—functions, dysfunctions and therapeutic targets. Nat. Rev. Gastroenterol. Hepatol. 10, 473–486 (2013).
Osteen, J. D. et al. Selective spider toxins reveal a role for the NaV1.1 channel in mechanical pain. Nature 534, 494–499 (2016).
Sadeghi, M. et al. Contribution of membrane receptor signalling to chronic visceral pain. Int. J. Biochem. Cell Biol. 98, 10–23 (2018).
Lu, V. B., Gribble, F. M. & Reimann, F. Free fatty acid receptors in enteroendocrine cells. Endocrinology 159, 2826–2835 (2018).
Mars, R. A. T. et al. Longitudinal multi-omics reveals subset-specific mechanisms underlying irritable bowel syndrome. Cell 182, 1460–1473 (2020).
Alcaino, C. et al. A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proc. Natl Acad. Sci. USA 115, e7632–e7641 (2018).
Wang, F. et al. Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J. Physiol. 595, 79–91 (2017).
Brierley, S. M., Jones, R. C. 3rd, Gebhart, G. F. & Blackshaw, L. A. Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology 127, 166–178 (2004).
Daou, I. et al. Remote optogenetic activation and sensitization of pain pathways in freely moving mice. J. Neurosci. 33, 18631–18640 (2013).
Kim, J. C. et al. Linking genetically defined neurons to behavior through a broadly applicable silencing allele. Neuron 63, 305–315 (2009).
Jensen, P. et al. Redefining the serotonergic system by genetic lineage. Nat. Neurosci. 11, 417–419 (2008).
Erspamer, V. & Asero, B. Identification of enteramine, the specific hormone of the enterochromaffin cell system, as 5-hydroxytryptamine. Nature 169, 800–801 (1952).
Spohn, S. N. & Mawe, G. M. Non-conventional features of peripheral serotonin signalling—the gut and beyond. Nat. Rev. Gastroenterol. Hepatol. 14, 412–420 (2017).
Brierley, S. M., Hibberd, T. J. & Spencer, N. J. Spinal afferent innervation of the colon and rectum. Front. Cell Neurosci. 12, 467 (2018).
Uhlig, F. et al. Identification of a quorum sensing-dependent communication pathway mediating bacteria–gut–brain cross talk. iScience 23, 101695 (2020).
Makadia, P. A. et al. Optogenetic activation of colon epithelium of the mouse produces high-frequency bursting in extrinsic colon afferents and engages visceromotor responses. J. Neurosci. 38, 5788–5798 (2018).
Grundy, L. et al. Chronic linaclotide treatment reduces colitis-induced neuroplasticity and reverses persistent bladder dysfunction. JCI Insight 3, e121841 (2018).
Najjar, S. A. et al. Optogenetic inhibition of the colon epithelium reduces hypersensitivity in a mouse model of inflammatory bowel disease. Pain 162, 1126–1134 (2021).
Jones, R. C. 3rd, Xu, L. & Gebhart, G. F. The mechanosensitivity of mouse colon afferent fibers and their sensitization by inflammatory mediators require transient receptor potential vanilloid 1 and acid-sensing ion channel 3. J. Neurosci. 25, 10981–10989 (2005).
Castro, J. et al. Activation of pruritogenic TGR5, MrgprA3, and MrgprC11 on colon-innervating afferents induces visceral hypersensitivity. JCI Insight 4, e131712 (2019).
Fothergill, L. J. & Furness, J. B. Diversity of enteroendocrine cells investigated at cellular and subcellular levels: the need for a new classification scheme. Histochem. Cell Biol. 150, 693–702 (2018).
Koo, A., Fothergill, L. J., Kuramoto, H. & Furness, J. B. 5-HT containing enteroendocrine cells characterised by morphologies, patterns of hormone co-expression, and relationships with nerve fibres in the mouse gastrointestinal tract. Histochem. Cell Biol. 155, 623–636 (2021).
Lumsden, A. L. et al. Sugar responses of human enterochromaffin cells depend on gut region, sex, and body mass. Nutrients 11, 234 (2019).
Bohórquez, D. V. et al. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J. Clin. Invest. 125, 782–786 (2015).
Brenner, D. M. & Sayuk, G. S. Current US Food and Drug Administration-approved pharmacologic therapies for the treatment of irritable bowel syndrome with diarrhea. Adv. Ther. 37, 83–96 (2020).
Bradesi, S. et al. Dual role of 5-HT3 receptors in a rat model of delayed stress-induced visceral hyperalgesia. Pain 130, 56–65 (2007).
Miranda, A., Peles, S., McLean, P. G. & Sengupta, J. N. Effects of the 5-HT3 receptor antagonist, alosetron, in a rat model of somatic and visceral hyperalgesia. Pain 126, 54–63 (2006).
El-Ayache, N. & Galligan, J. J. 5-HT3 receptor signaling in serotonin transporter-knockout rats: a female sex-specific animal model of visceral hypersensitivity. Am. J. Physiol. Gastrointest. Liver Physiol. 316, G132–G143 (2019).
Hicks, G. A. et al. Excitation of rat colonic afferent fibres by 5-HT(3) receptors. J. Physiol. 544, 861–869 (2002).
Ji, Y., Tang, B. & Traub, R. J. The visceromotor response to colorectal distention fluctuates with the estrous cycle in rats. Neuroscience 154, 1562–1567 (2008).
Gustafsson, J. K. & Greenwood-Van Meerveld, B. Amygdala activation by corticosterone alters visceral and somatic pain in cycling female rats. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G1080–G1085 (2011).
Ji, Y., Murphy, A. Z. & Traub, R. J. Estrogen modulates the visceromotor reflex and responses of spinal dorsal horn neurons to colorectal stimulation in the rat. J. Neurosci. 23, 3908–3915 (2003).
Balasuriya, G. K., Hill-Yardin, E. L., Gershon, M. D. & Bornstein, J. C. A sexually dimorphic effect of cholera toxin: rapid changes in colonic motility mediated via a 5-HT3 receptor-dependent pathway in female C57Bl/6 mice. J. Physiol. 594, 4325–4338 (2016).
Törnblom, H. & Drossman, D. A. Psychopharmacologic therapies for irritable bowel syndrome. Gastroenterol. Clin. North Am. 50, 655–669 (2021).
Galligan, J. J. et al. Visceral hypersensitivity in female but not in male serotonin transporter knockout rats. Neurogastroenterol. Motil. 25, e373–e381 (2013).
Wang, Y. C. et al. The ETS oncogene family transcription factor FEV identifies serotonin-producing cells in normal and neoplastic small intestine. Endocr. Relat. Cancer 17, 283–291 (2010).
Hennessy, M. L. et al. Activity of Tachykinin1-expressing Pet1 raphe neurons modulates the respiratory chemoreflex. J. Neurosci. 37, 1807–1819 (2017).
Madison, B. B. et al. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J. Biol. Chem. 277, 33275–33283 (2002).
Salvatierra, J. et al. NaV1.1 inhibition can reduce visceral hypersensitivity. JCI Insight 3, e121000 (2018).
Hockley, J. R. F. et al. Single-cell RNAseq reveals seven classes of colonic sensory neuron. Gut 68, 633–644 (2019).
Cantu, D. A. et al. EZcalcium: open-source toolbox for analysis of calcium imaging data. Front. Neural Circuits 14, 25 (2020).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Sato, T. et al. Single Lgr5 stem cells build crypt–villus structures in vitro without a mesenchymal niche. Nature 459, 262–265 (2009).
Becker, L. et al. Age-dependent shift in macrophage polarisation causes inflammation-mediated degeneration of enteric nervous system. Gut 67, 827–836 (2018).
Li, Z. S., Schmauss, C., Cuenca, A., Ratcliffe, E. & Gershon, M. D. Physiological modulation of intestinal motility by enteric dopaminergic neurons and the D2 receptor: analysis of dopamine receptor expression, location, development, and function in wild-type and knock-out mice. J. Neurosci. 26, 2798–2807 (2006).
Acknowledgements
We thank J. Poblete for expert assistance with animal husbandry and genotyping; the Garvan Institute, Australia, for genotyping services; the Preclinical, Imaging and Research Laboratories (SAHMRI) for the use of their small animal facility; and the University of South Australia and the UCSF Nikon Imaging Core for use of their confocal imaging facilities. We thank K. Yackle and all members of our groups for many helpful suggestions and critical comments and N. Bellono for strategic contributions in the early stages of this project. We thank B. Yu for assistance in transmitter surgeries and animal care. This work was supported by NIH training grant T32 DK007762 and postdoctoral fellowship from the A.P. Giannini Foundation (R.D.M.) and the Damon Runyon Cancer Research Foundation (K.K.T.), a Simons Foundation Autism Research Initiative Pilot Award (514791 to D.J.), a Rainin Foundation Innovator Award (20191150 to D.J.), grants from the US National Institutes of Health (HEAL-SPARC Initiative U01NS113869 to H.A.I., D.J. and S.M.B., R35 NS105038 to D.J., R01 DK121657 and GCRLE0320 to H.A.I., and R03 DK121061 and R01 DK128346 to J.R.B.), National Health and Medical Research Council of Australia (NHMRC) Investigator Leadership Grant (APP2008727 to S.M.B.), an NHMRC Development Grant (APP2014250 to S.M.B), an NHMRC Ideas Grant (APP1181448 to J.C.) and the Hospital Research Foundation PhD scholarship (SAPhD000242018 to J.M.).
Author information
Authors and Affiliations
Contributions
R.D.M. developed and implemented transgenic strategies for targeting DREADD and PfTox expression to EC cells. N.D.R., A.V. and R.D.M. characterized DREADD and PfTox expression in these lines histologically. J.C. and S.M.B. designed, performed and analysed ex vivo studies mechanically and optogenetically stimulating colonic mucosal afferents. M.B. and S.M.B. designed, performed and analysed ex vivo studies on colonic distension-sensitive afferents. M.B. performed and analysed in vitro responses of NaV1.8-GCaMP6 DRG neurons. G.S., J.M., J.C., S.M.B., K.B. and J.R.B. designed, performed and analysed VMR to CRD. J.C., A.H. and S.M.B. designed, performed and analysed intravital GCaMP imaging studies. S.G.-C. quantified eYFP expression in male and female NaV1.8-Ch2 DRG neurons. K.K.T. quantified serotonin release from organoids. A.V., F.C.N. and C.B.S. collected blood samples and measured serotonin levels in serum. A.V. performed and analysed experiments to assess anxiety-related behaviours. H.A.I., J.R.B., S.M.B. and D.J. wrote the manuscript with input and suggestions from all authors and provided advice and guidance throughout.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Sex-specific sensitization of mucosal afferents and visceromotor responses by isovalerate.
a, Graphical scheme of ex vivo mucosal afferent recordings (evMAR) from NaV1.8-ChR2 mice. b-c, Representative pelvic mucosal afferent fibre responses showing that isovalerate lowers the threshold to optogenetic activation (0.05–34 mW/mm2) in males whereas females display heightened baseline sensitivity. d, Fluorescent channelrhodopsin-eYFP signal in L6 DRG sections from NaV1.8-ChR2 mice, showing no difference between sexes. Scale bars = 100 μm. e, Heatmaps from two representative males showing in vivo NaV1.8-GCaMP6s responses of L6 DRG neurons to intracolonic application of an isovalerate bolus (200 μM) after pretreatment with alosetron (10 μM). f, Responses of dissociated DRG neurons from male (N = 3) and female (N = 3) NaV1.8-GCaMP6s mice showing no sex differences in responsiveness to ionomycin 5 μM (females: 514 neurons from 15 coverslips, males: 521 neurons from 18 coverslips), potassium chloride (KCl 30 mM; females: 478 neurons from 11 coverslips, males: 629 neurons from 18 coverslips) or capsaicin 20 nM (females: 246 neurons from 11 coverslips, males: 363 neurons from 18 coverslips). g, Data from individual mice showing total area under the curve for all colonic distension pressures. VMRs are significantly increased in male but not female mice following intracolonic application of isovalerate (100 μl bolus of 200 μM) versus vehicle; male: N = 12 vehicle, N = 12 isovalerate, female: N = 12 vehicle, N = 12 isovalerate). h, total AUC compared across cohorts, similarly demonstrating increased VMRs over baseline (grey) following application of isovalerate (purple) in male but not female mice (male: N = 15 vehicle, N = 9 isovalerate, female: N = 7 vehicle, N = 7 isovalerate). i, Colonic compliance is unchanged with isovalerate in both male and female mice. Unpaired t-test, 2-tailed in panel d, Unpaired Mann–Whitney U test, 2-tailed in panels f, h, Wilcoxon matched-pairs signed-rank test 2-tailed in panel g and two-way ANOVA in panel i. **p < 0.01, ***p < 0.001, error bars represent mean ± SEM. N = number of animals and n = number of neurons/sections. The graphical element in panel a was created using BioRender (https://biorender.com).
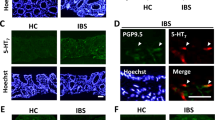
Extended Data Fig. 2 Intersectional genetic strategy targets gene expression to gut EC cells.
a, Histologic sections of ECPFTox (Pet1Flp;Tac1Cre;RC::PFTox) colon demonstrating colocalization of PFTox allele GFP reporter (green) and 5-HT (magenta); representative of 93 fields from at least 2 animals Scale bars = 50 μm (upper) and 10 μm (lower). Colon EC cell density is unchanged by expression of PFTox allele (n = 6, 6 independent fields). b, Histologic sections of EChM3Dq (Pet1Flp;Tac1Cre;RC::FL-hM3Dq) colon and duodenum demonstrating colocalization (white arrowheads) of the hM3Dq allele reporter (red) and 5-HT (magenta); representative of 129 fields from at least 2 animals. Scale bars=50 μm (upper) and 10 μm (lower). Colon EC cell density is unchanged following three weeks of DREADD agonist treatment (n = 6, 6 independent fields). c, Whole-mount small intestine (jejunum) of EChM3Dq demonstrating expression of single-(Pet1Flp-GFP, green) and double-recombination (Pet1Flp::Tac1Cre, mCherry, red) hM3Dq allele reporters and 5-HT (magenta); representative of 10 fields from at least 2 animals. Scale bar 50 μm. d, Whole-mount DRG from spinal segments L6/S1 of EChM3Dq demonstrating the absence of mCherry (red) expression; representative of 8 fields from 1 animal. Scale bars = 50 μm. e, Images of EChM3Dq dorsal raphe (DR) and median raphe (MnR) nuclei showing 5-HT-expressing neurons (yellow) and lack of mCherry (red) expression; representative of 6-8 fields from each of 3 animals. Scale bar = 100 μm. f, Images of EChM3Dq lumbosacral spinal cord (L6-S1) showing the absence of mCherry (red) and 5-HT (yellow) expression; representative of 10 fields from each of 3 animals. Scale bar = 100 µm. g, Quantitation of specificity and penetrance of intersectional genetic approach demonstrating ≥ 95% (Tac1Cre, light grey) and 80% (Vil1Cre, dark grey) EC cell specificity and ~60% penetrance in the colon from male mice (data collected from 20–30 random fields). Student’s t-test (unpaired, 2-tailed) in panels a, b; ns = not significant, error bars represent mean ± SEM.
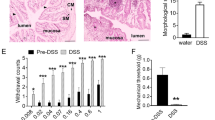
Extended Data Fig. 3 Silencing EC cells attenuates responses to irritants and noxious colonic distension.
a, Representative examples of pelvic mucosal afferents firing more action potentials in response to stroking with 10 mg or 500 mg von frey hairs (vfh) in the presence of isovalerate for control (TacCre, left panel) but not ECPFTox (right panel) mice. b,c, Group data showing before and after isovalerate (200 μM) application response to increasing mechanical stimulation with vfh for males (b) and females (c) for control (TacCre, upper panels) and ECPFTox (lower panels) mice. d, Group data showing total area under the curve for all colonic distension pressures showing VMRs significantly reduced in ECPFTox versus TacCre males (N = 6, 12) and females (N = 9, 8). e, Colonic compliance is unchanged in ECPFTox animals. Wilcoxon matched-pairs signed-rank 2-tailed test in panels b, c; unpaired 2-tailed Mann–Whitney U test for panel d; two-way ANOVA in panel e. *p < 0.05, **p < 0.01, ns = not significant, error bars represent mean ± SEM. N = number of animals, n = number of afferents.
Extended Data Fig. 4 Activating EC cells increases afferent output and VMR to colorectal distension.
a, DREADD agonist DCZ (1.7 μM) elicits Ca2+ responses in EChM3Dq intestinal organoids as detected by a change in GCaMP fluorescence ratio. b, Representative examples of pelvic mucosal afferents firing action potentials in response to stroking with 10 mg or 500 mg von frey hairs (vfh) in the presence of vehicle (black/grey) or CNO (100 μM; green) for control (upper panels) and EChM3Dq (lower panels) mice. c,d, Group data showing before and after CNO (100 μM) application response to increasing mechanical stimulation with vfh for males (c) and females (d) for control (TacCre upper panels) and EChM3Dq (lower panels) mice. e, Group data showing total area under the curve for all colonic distension pressures showing VMRs significantly increased in TacCre and EChM3Dq male mice (N = 6, 7) following DCZ (75 μg/kg i.p.). f, Colonic compliance is unchanged in EChM3Dq animals. Wilcoxon matched-pairs signed-rank 2-tailed test in panels c, d; Student’s t-test (unpaired, 2-tailed) in panel e; two-way ANOVA in panel f. *p < 0.05, **p < 0.01, ns = not significant, error bars represent mean ± SEM. N = number of animals and n = number of afferents.
Extended Data Fig. 5 Gastrointestinal transit is unchanged in EC manipulation models.
a, Total gastrointestinal and colonic transit times are similar between TacCre control (black) and ECPFTox (red) male (N = 5, 3) and female (N = 5, 4) mice. b, Total gastrointestinal transit times trend faster in DCZ-treated (75 μg/kg) TacCre and EChM3Dq male (N = 14, 8) and female (N = 4, 4) mice, as did colonic transit times for male (N = 3, 4) and female (N = 7, 7) mice. Transit measurements started 15 min after DCZ i.p. injection. Unpaired 2-tailed Mann–Whitney U test in panels a, b. ns = not significant. N = number of animals.
Extended Data Fig. 6 EC cells do not modulate distension-sensitive afferents.
Group data showing afferent firing to increasing distension pressures in colonic preparations from VilCre control mice at baseline and following isovalerate. Two-way ANOVA (Šidák’s multiple-comparisons test); ns = not significant, error bars represent mean ± SEM. n = number of afferents.
Extended Data Fig. 7 Activation and silencing of EC cells in male and female mice increase anxiety-like behaviours.
a, Time spent in open or closed arms of EPM following DCZ treatment (75 μg/kg i.p.) 10 min before testing. The total time mobile and total distance travelled remain unchanged between TacCre control and EChM3Dq mice (N = 12, 9). b, Time spent in open or closed arms of EPM for ECPFTox and TacCre control animals (N = 18, 12). The total time mobile and total distance travelled remain unchanged between ECPFTox and TacCre animals (N = 18, 12). c, ECPFTox mice show significantly reduced marble-burying behaviour compared to TacCre controls (N = 17, 12). d, ECPFTox mice do not show differences in nestlet shredding behaviour (N = 14, 12). e, Contextual and cued fear conditioning in TacCre and ECPFTox mice (N = 9, 8). Two-way ANOVA (Šidák’s multiple-comparisons test) for panel a. Unpaired 2-tailed Mann–Whitney test for panels b–e. **p < 0.01, ns = not significant, error bars represent mean ± SEM.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bayrer, J.R., Castro, J., Venkataraman, A. et al. Gut enterochromaffin cells drive visceral pain and anxiety. Nature 616, 137–142 (2023). https://doi.org/10.1038/s41586-023-05829-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-023-05829-8
This article is cited by
-
Kalium channelrhodopsins effectively inhibit neurons
Nature Communications (2024)
-
The crosstalk between enteric nervous system and immune system in intestinal development, homeostasis and diseases
Science China Life Sciences (2024)
-
The intestine as an endocrine organ and the role of gut hormones in metabolic regulation
Nature Reviews Gastroenterology & Hepatology (2023)
-
Enterochromaffin Cell: Friend or Foe for Human Health?
Neuroscience Bulletin (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.