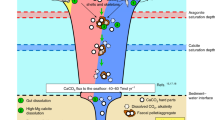
Abstract
Calcium carbonate formation is the primary pathway by which carbon is returned from the ocean–atmosphere system to the solid Earth1,2. The removal of dissolved inorganic carbon from seawater by precipitation of carbonate minerals—the marine carbonate factory—plays a critical role in shaping marine biogeochemical cycling1,2. A paucity of empirical constraints has led to widely divergent views on how the marine carbonate factory has changed over time3,4,5. Here we use geochemical insights from stable strontium isotopes to provide a new perspective on the evolution of the marine carbonate factory and carbonate mineral saturation states. Although the production of carbonates in the surface ocean and in shallow seafloor settings have been widely considered the predominant carbonate sinks for most of the history of the Earth6, we propose that alternative processes—such as porewater production of authigenic carbonates—may have represented a major carbonate sink throughout the Precambrian. Our results also suggest that the rise of the skeletal carbonate factory decreased seawater carbonate saturation states.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available in the main text or the Supplementary Information. All data are also reposited in EarthChem (https://doi.org/10.26022/IEDA/112713).
Code availability
We used the open-source language R (version 4.1.1) to analyse the measured data, analyse the EarthChem (http://portal.earthchem.org/) and Macrostrat (https://macrostrat.org/#api) datasets, and generate all plots. All equations for the mass-balance model are listed in the Supplementary Information and all associated code is deposited on GitHub (https://github.com/julianwangnwu/carbonatefactoryevolution).
References
Ridgwell, A. & Zeebe, R. E. The role of the global carbonate cycle in the regulation and evolution of the Earth system. Earth Planet. Sci. Lett. 234, 299–315 (2005).
Isson, T. T. et al. Evolution of the global carbon cycle and climate regulation on Earth. Global Biogeochem. Cycles 34, 1–28 (2020).
Wilkinson, B. H. & Walker, J. C. G. Phanerozoic cycling of sedimentary carbonate. Am. J. Sci. 289, 525–548 (1989).
Ridgwell, A. A Mid Mesozoic Revolution in the regulation of ocean chemistry. Mar. Geol. 217, 339–357 (2005).
Higgins, J. A., Fischer, W. W. & Schrag, D. P. Oxygenation of the ocean and sediments: consequences for the seafloor carbonate factory. Earth Planet. Sci. Lett. 284, 25–33 (2009).
James, N. P. & Jones, B. Origin of Carbonate Sedimentary Rocks (Wiley, 2015).
Schlager, W. Sedimentation rates and growth potential of tropical, cool-water and mud-mound carbonate systems. Geol. Soc. Spec. Publ. 178, 217–227 (2000).
Schrag, D. P., Higgins, J. A., Macdonald, F. A. & Johnston, D. T. Authigenic carbonate and the history of the global carbon cycle. Science 339, 540–543 (2013).
Gilbert, P., Bergmann, K. & Knoll, A. H. Biomineralization: integrating mechanism and evolutionary history. Sci. Adv. 8, eabl9653 (2021).
Grotzinger, J. P. & James, N. P. in Carbonate Sedimentation and Diagenesis in the Evolving Precambrian World 3–20 (SEPM Society for Sedimentary Geology, 2000).
Cantine, M. D., Knol, A. H. & Bergmann, K. D. Carbonates before skeletons: a database approach. Earth-Sci. Rev. 201, 103065 (2020).
Simonson, B. M., Schubel, K. A. & Hassler, S. W. Carbonate sedimentology of the early Precambrian Hamersley Group of Western Australia. Precambrian Res. 60, 287–335 (1993).
Grotzinger, J. P. Geochemical model for Proterozoic stromatolite decline. Am. J. Sci. 290, 80–103 (1990).
Vollstaedt, H. et al. The Phanerozoic δ88/86Sr record of seawater: new constraints on past changes in oceanic carbonate fluxes. Geochim. Cosmochim. Acta 128, 249–265 (2014).
Wang, J., Jacobson, A. D., Sageman, B. B. & Hurtgen, M. T. Stable Ca and Sr isotopes support volcanically triggered biocalcification crisis during Oceanic Anoxic Event 1a. Geology 49, 515–519 (2021).
Paytan, A. et al. A 35-million-year record of seawater stable Sr isotopes reveals a fluctuating global carbon cycle. Science 371, 1346–1350 (2021).
Böhm, F. et al. Strontium isotope fractionation of planktic foraminifera and inorganic calcite. Geochim. Cosmochim. Acta 93, 300–314 (2012).
AlKhatib, M. & Eisenhauer, A. Calcium and strontium isotope fractionation in aqueous solutions as a function of temperature and reaction rate; I. Calcite. Geochim. Cosmochim. Acta 209, 296–319 (2017).
Müller, M. N., Krabbenhöft, A., Vollstaedt, H., Brandini, F. P. & Eisenhauer, A. Stable isotope fractionation of strontium in coccolithophore calcite: influence of temperature and carbonate chemistry. Geobiology 16, 297–306 (2018).
Stevenson, E. I. et al. Controls on stable strontium isotope fractionation in coccolithophores with implications for the marine Sr cycle. Geochim. Cosmochim. Acta 128, 225–235 (2014).
Kalderon-Asael, B. et al. A lithium-isotope perspective on the evolution of carbon and silicon cycles. Nature 595, 394–398 (2021).
Banner, J. L. Application of the isotope and trace element geochemistry of strontium to studies of diagenesis in carbonate systems. Sedimentology 42, 805–824 (1995).
Shields, G. & Veizer, J. Precambrian marine carbonate isotope database: version 1.1. Geochem. Geophys. Geosyst. 3, 10.1029/2001GC000266 (2002).
Wang, J. et al. Coupled δ44/40Ca, δ88/86Sr, and 87Sr/86Sr geochemistry across the end-Permian mass extinction event. Geochim. Cosmochim. Acta 262, 143–165 (2019).
Wang, J., Jacobson, A. D., Sageman, B. B. & Hurtgen, M. T. δ44/40Ca-δ88/86Sr multi-proxy constrains primary origin of Marinoan cap carbonates. Preprint at https://arxiv.org/abs/2204.02563 (2022).
Kump, L., Bralower, T. & Ridgwell, A. Ocean acidification in deep time. Oceanography 22, 94–107 (2009).
Moynier, F., Agranier, A., Hezel, D. C. & Bouvier, A. Sr stable isotope composition of Earth, the Moon, Mars, Vesta and meteorites. Earth Planet. Sci. Lett. 300, 359–366 (2010).
Dupraz, C. et al. Processes of carbonate precipitation in modern microbial mats. Earth-Sci. Rev. 96, 141–162 (2009).
Dunne, J. P., Sarmiento, J. L. & Gnanadesikan, A. A synthesis of global particle export from the surface ocean and cycling through the ocean interior and on the seafloor. Global Biogeochem. Cycles 21, GB4006 (2007).
Wright, V. P. & Cherns, L. Leaving no stone unturned: the feedback between increased biotic diversity and early diagenesis during the Ordovician. J. Geol. Soc. 173, 241–244 (2016).
Laakso, T. A. & Schrag, D. P. The role of authigenic carbonate in Neoproterozoic carbon isotope excursions. Earth Planet. Sci. Lett. 549, 116534 (2020).
Blättler, C. L. & Higgins, J. A. Testing Urey’s carbonate–silicate cycle using the calcium isotopic composition of sedimentary carbonates. Earth Planet. Sci. Lett. 479, 241–251 (2017).
Weiner, S. & Dove, P. M. An overview of biomineralization processes and the problem of the vital effect. Rev. Mineral. Geochem. 54, 1–29 (2003).
Ronov, A. B., Khain, V. E., Balukhovsky, A. N. & Seslavinsky, K. B. Quantitative analysis of Phanerozoic sedimentation. Sediment. Geol. 25, 311–325 (1980).
Krissansen-Totton, J., Buick, R. & Catling, D. C. A statistical analysis of the carbon isotope record from the Archean to Phanerozoic and implications for the rise of oxygen. Am. J. Sci. 315, 275–316 (2015).
Liu, C., Wang, Z. & Raub, T. D. Geochemical constraints on the origin of Marinoan cap dolostones from Nuccaleena Formation, South Australia. Chem. Geol. 351, 95–104 (2013).
Wang, J., Asael, D., Planavsky, N. J. & Tarhan, L. G. An investigation of factors affecting high-precision Sr isotope analyses (87Sr/86Sr and δ88/86Sr) by MC-ICP-MS. Preprint at https://arxiv.org/abs/2111.02942 (2021).
Ohno, T. & Hirata, T. Simultaneous determination of mass-dependent isotopic fractionation and radiogenic isotope variation of strontium in geochemical samples by multiple collector-ICP-mass spectrometry. Anal. Sci. 23, 1275–1280 (2007).
Ma, J. L. et al. Precise measurement of stable (δ88/86Sr) and radiogenic (87Sr/86Sr) strontium isotope ratios in geological standard reference materials using MC-ICP-MS. Chin. Sci. Bull. 58, 3111–3118 (2013).
Andrews, M. G., Jacobson, A. D., Lehn, G. O., Horton, T. W. & Craw, D. Radiogenic and stable Sr isotope ratios (87Sr/86Sr, δ88/86Sr) as tracers of riverine cation sources and biogeochemical cycling in the Milford Sound region of Fiordland, New Zealand. Geochim. Cosmochim. Acta 173, 284–303 (2016).
Machel, H. G. in Cathodoluminescence in Geosciences 271–301 (Springer, 2000).
Bathurst, R. G. C. Carbonate Sediments and Their Diagenesis (Elsevier, 1972).
Brand, U. & Veizer, J. Chemical diagenesis of a multicomponent carbonate system; 1, trace elements. J. Sediment. Res. 50, 1219–1236 (1980).
Anderson, T. F. & Arthur, M. A. in Stable Isotopes in Sedimentary Geology (eds Arthur, M. A., Anderson, T. F., Kaplan, I. R., Veizer, J. & Land, L. S.) (SEPM Society for Sedimentary Geology, 1983).
Richter, F. M. & DePaolo, D. J. Diagenesis and Sr isotopic evolution of seawater using data from DSDP 590B and 575. Earth Planet. Sci. Lett. 90, 382–394 (1988).
Richter, F. M. & Liang, Y. The rate and consequences of Sr diagenesis in deep-sea carbonates. Earth Planet. Sci. Lett. 117, 553–565 (1993).
Holland, H., Holland, H. & Munoz, J. The coprecipitation of cations with CaCO3—II. The coprecipitation of Sr+2 with calcite between 90° and 100°C. Geochim. Cosmochim. Acta 28, 1287–1301 (1964).
Katz, A., Sass, E., Starinsky, A. & Holland, H. D. Strontium behavior in the aragonite-calcite transformation: an experimental study at 40–98°C. Geochim. Cosmochim. Acta 36, 481–496 (1972).
Kinsman, D. J. J. & Holland, H. D. The co-precipitation of cations with CaCO3—IV. The co-precipitation of Sr2+ with aragonite between 16° and 96°C. Geochim. Cosmochim. Acta 33, 1–17 (1969).
Derry, L. A., Kaufman, A. J. & Jacobsen, S. B. Sedimentary cycling and environmental change in the Late Proterozoic: evidence from stable and radiogenic isotopes. Geochim. Cosmochim. Acta 56, 1317–1329 (1992).
Cruse, A. M. & Lyons, T. W. Trace metal records of regional paleoenvironmental variability in Pennsylvanian (Upper Carboniferous) black shales. Chem. Geol. 206, 319–345 (2004).
Sageman, B. B. et al. A tale of shales: the relative roles of production, decomposition, and dilution in the accumulation of organic-rich strata, Middle-Upper Devonian, Appalachian basin. Chem. Geol. 195, 229–273 (2003).
Piper, D. Z. & Calvert, S. E. A marine biogeochemical perspective on black shale deposition. Earth-Sci. Rev. 95, 63–96 (2009).
Banner, J. L. & Hanson, G. N. Calculation of simultaneous isotopic and trace element variations during water-rock interaction with applications to carbonate diagenesis. Geochim. Cosmochim. Acta 54, 3123–3137 (1990).
Baker, P. A., Gieskes, J. M. & Elderfield, H. Diagenesis of carbonates in deep-sea sediments; evidence from Sr/Ca ratios and interstitial dissolved Sr2+ data. J. Sediment. Res. 52, 71–82 (1982).
Chaudhuri, S. & Clauer, N. Strontium isotopic compositions and potassium and rubidium contents of formation waters in sedimentary basins: clues to the origin of the solutes. Geochim. Cosmochim. Acta 57, 429–437 (1993).
Stueber, A. M., Pushkar, P. & Hetherington, E. A. A strontium isotopic study of Smackover brines and associated solids, southern Arkansas. Geochim. Cosmochim. Acta 48, 1637–1649 (1984).
McNutt, R. H., Frape, S. K., Fritz, P., Jones, M. G. & MacDonald, I. M. The 87Sr/86Sr values of Canadian Shield brines and fracture minerals with applications to groundwater mixing, fracture history, and geochronology. Geochim. Cosmochim. Acta 54, 205–215 (1990).
McNutt, R. H., Frape, S. K. & Fritz, P. Strontium isotopic composition of some brines from the Precambrian Shield of Canada. Chem. Geol. 46, 205–215 (1984).
Wilcots, J., Gilbert, P. U. P. A. & Bergmann, K. D. Nanoscale crystal fabric of primary Ediacaran dolomite. Preprint at ESS Open Archive https://doi.org/10.1002/essoar.10507750.1 (2021).
Ohno, T., Komiya, T., Ueno, Y., Hirata, T. & Maruyama, S. Determination of 88Sr/86Sr mass-dependent isotopic fractionation and radiogenic isotope variation of 87Sr/86Sr in the Neoproterozoic Doushantuo Formation. Gondwana Res. 14, 126–133 (2008).
Sawaki, Y. et al. Sr isotope excursion across the Precambrian–Cambrian boundary in the Three Gorges area, South China. Gondwana Res. 14, 134–147 (2008).
Pearce, C. R. et al. Reassessing the stable (δ88/86Sr) and radiogenic (87Sr/86Sr) strontium isotopic composition of marine inputs. Geochim. Cosmochim. Acta 157, 125–146 (2015).
McArthur, J. M., Howarth, R. J. & Shields, G. A. in The Geologic Time Scale (eds Gradstein, F. M., Ogg, J. G., Schmitz, M. & Ogg, G. M.) 127–144 (Elsevier, 2012).
Acknowledgements
We thank S. Nicolescu, B. Kalderon-Asael and Y. Wang for facilitating access to the Yale Peabody Museum and Woods Hole Oceanographic Institution collections and for assistance with sample selection; D. Asael for assistance with MC-ICP-MS method development; R. P. Reid and E. P. Suosaari for access to the Hamelin Pool stromatolite samples; S. Ye for assistance with the Macrostrat database; and D. Schrag, M. Arthur, K. Bergmann, Z. Zhang and Y. Cui for helpful discussions. This study is supported by an Agouron Geobiology Postdoctoral Fellowship to J.W. and National Aeronautics and Space Administration Interdisciplinary Consortia for Astrobiology Research grant (NNA15BB03A) to N.J.P.
Author information
Authors and Affiliations
Contributions
J.W., L.G.T. and N.J.P. conceived the study and acquired funding. J.W., L.G.T., A.D.J. and N.J.P. developed the methodology. J.W. performed mass spectrometry analyses. J.W. and L.G.T. conducted the statistical analyses. J.W. and L.G.T. wrote the paper, with input from A.D.J., A.M.O. and N.J.P. J.W., L.G.T., A.D.J., A.M.O. and N.J.P. all contributed to the interpretation of the results and editing the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Adina Paytan and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Marine carbonate strontium isotope records through the history of the Earth.
a, Summary of δ88/86Sr values measured in marine calcites and dolomites. New data from this study (n = 139) are colour-contoured to indicate corresponding radiogenic Sr isotope ratios (87Sr/86Sr) generated from the same samples: circles, calcite; diamonds, dolomite; ×, calcite with abnormally high 87Sr/86Sr ratios. Error bars represent the long-term external reproducibility of δ88/86Sr (2σSD = ±0.03‰, n = 273). Purple crosses denote duplicate measurements of the same sample (see Methods for description of duplicate strategy). The gold line illustrates the δ88/86Sr value of bulk silicate Earth (0.27‰)27. The dashed blue line represents the δ88/86Sr value of modern marine carbonate63. Other symbols represent published data from other studies (n = 299; see Methods): pink squares, non-skeletal carbonate; grey gridded squares, cap carbonate; grey triangles, bulk skeletal calcite; grey crosses, belemnite; grey inverted triangles, brachiopod. b, Marine carbonate 87Sr/86Sr ratios. New data from this study are denoted by coloured symbols: circles, calcite; diamonds, dolomite; ×, calcite with abnormally high 87Sr/86Sr ratios. The grey circles represent Precambrian carbonate 87Sr/86Sr records (n = 1,494)23. The dashed curve denotes the LOESS fit of the lowest 10% of Precambrian 87Sr/86Sr ratios23 and the solid curve denotes the LOESS fit of the Phanerozoic 87Sr/86Sr record64.
Extended Data Fig. 2 Histograms of measured and bootstrap-resampled Precambrian calcite and dolomite δ88/86Sr values.
a, Measured Precambrian calcite (red) and dolomite (yellow) δ88/86Sr values. b, Bootstrap-resampled (n = 10,000) Precambrian calcite (red) and dolomite (yellow) δ88/86Sr values. All Precambrian calcite and dolomite δ88/86Sr values are from this study. The purple and green curves represent density distributions of δ88/86Sr in Precambrian calcites (purple, n = 72) and dolomites (green, n = 43).
Extended Data Fig. 3 The relationship between δ88/86Sr and 87Sr/86Sr in analysed dolomites.
a, The stable and radiogenic Sr isotope relationship for all analysed dolomites (n = 43). A SMA regression model yields R2 = 0.223 and P = 0.001. b, The stable and radiogenic Sr isotopic values of less-altered dolomite samples from this dataset, that is, samples characterized by 87Sr/86Sr values less than 0.708, the inferred value of Ediacaran seawater64.
Extended Data Fig. 4 Cross-plots of δ88/86Sr versus different elemental contents and ratios.
a, δ88/86Sr versus CaCO3 weight percentage (wt%). Carbonate wt% calculated using calcium content assuming stoichiometric CaCO3. b, δ88/86Sr versus Sr contents. c, δ88/86Sr versus Mn/Sr. d, δ88/86Sr versus Rb contents. e, δ88/86Sr versus Ti contents. f, δ88/86Sr versus Pb contents. δ88/86Sr values are given in ‰, normalized to NIST 987; all elemental concentrations are in ppm except when noted otherwise. An SMA regression model was used to evaluate the statistical significance of each correlation. R2 and P-values are listed at the top of each panel. No statistical correlations are observed at the significance level of 0.01.
Extended Data Fig. 5 Box plot of δ88/86Sr values for skeletal (green), microbial (red) and non-skeletal, non-microbial carbonate (blue) for modern, Permian–Triassic and Precambrian deposits.
In the box plot, the centre line represents the median of the data (50th percentile), box limits represent the upper and lower quartiles (75th and 25th percentiles), whiskers represent 1.5 times the interquartile range, blank points represent outliers and coloured points represent all data. These data indicate that the notably higher δ88/86Sr values characterizing Precambrian calcites cannot be attributed to differences between microbial and non-microbial pathways of carbonate precipitation and that, similarly, the shift between elevated Precambrian and less-elevated Phanerozoic δ88/86Sr values cannot be readily attributed to differences between skeletal and non-skeletal pathways of carbonate precipitation. The modern δ88/86Sr records are from Stevenson et al.20 (skeletal n = 10) and this study (microbial n = 5; non-skeletal, non-microbial n = 8); the Permian–Triassic δ88/86Sr records (skeletal n = 6; microbial n = 8; non-skeletal, non-microbial n = 20) are from Wang et al.24; the Precambrian calcite (microbial calcite n = 12; non-skeletal, non-microbial calcite n = 47) and dolomite (microbial dolomite n = 6; non-skeletal, non-microbial dolomite n = 37) δ88/86Sr records are from this study.
Supplementary information
Supplementary Information
This file contains Supplementary Discussion, Supplementary References and Supplementary Figs. 1 and 2.
Supplementary Table 1
Descriptions and geochemistry of analysed Precambrian carbonate samples.
Supplementary Table 2
Descriptions and Sr isotope ratios of analysed modern and palaeogene carbonate samples.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, J., Tarhan, L.G., Jacobson, A.D. et al. The evolution of the marine carbonate factory. Nature 615, 265–269 (2023). https://doi.org/10.1038/s41586-022-05654-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05654-5
This article is cited by
-
Crustal carbonate build-up as a driver for Earth’s oxygenation
Nature Geoscience (2024)
-
Myriad Mapping of nanoscale minerals reveals calcium carbonate hemihydrate in forming nacre and coral biominerals
Nature Communications (2024)
-
In-situ δ18O and 87Sr/86Sr proxies in an unconformable clastic unit at the Ordovician–Silurian transition
Scientific Reports (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.