Abstract
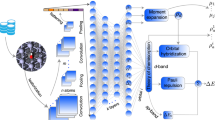
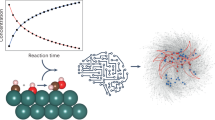
A mechanistic understanding of catalytic organic reactions is crucial for the design of new catalysts, modes of reactivity and the development of greener and more sustainable chemical processes1,2,3,4,5,6,7,8,9,10,11,12,13. Kinetic analysis lies at the core of mechanistic elucidation by facilitating direct testing of mechanistic hypotheses from experimental data. Traditionally, kinetic analysis has relied on the use of initial rates14, logarithmic plots and, more recently, visual kinetic methods15,16,17,18, in combination with mathematical rate law derivations. However, the derivation of rate laws and their interpretation require numerous mathematical approximations and, as a result, they are prone to human error and are limited to reaction networks with only a few steps operating under steady state. Here we show that a deep neural network model can be trained to analyse ordinary kinetic data and automatically elucidate the corresponding mechanism class, without any additional user input. The model identifies a wide variety of classes of mechanism with outstanding accuracy, including mechanisms out of steady state such as those involving catalyst activation and deactivation steps, and performs excellently even when the kinetic data contain substantial error or only a few time points. Our results demonstrate that artificial-intelligence-guided mechanism classification is a powerful new tool that can streamline and automate mechanistic elucidation. We are making this model freely available to the community and we anticipate that this work will lead to further advances in the development of fully automated organic reaction discovery and development.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated for training, validation and testing are available from figshare: https://doi.org/10.48420/16965292.
Code availability
Trained models, weights and python scripts are available from https://doi.org/10.48420/16965271.
References
Simonetti, M., Cannas, D. M., Just-Baringo, X., Vitorica-Yrezabal, I. J. & Larrosa, I. Cyclometallated ruthenium catalyst enables late-stage directed arylation of pharmaceuticals. Nat. Chem. 10, 724–731 (2018).
Salazar, C. A. et al. Tailored quinones support high-turnover Pd catalysts for oxidative C-H arylation with O2. Science 370, 1454–1460 (2020).
DiRocco, D. A. et al. A multifunctional catalyst that stereoselectively assembles prodrugs. Science 356, 426–430 (2017).
Li, T. et al. Efficient, chemoenzymatic process for manufacture of the Boceprevir bicyclic [3.1.0]proline intermediate based on amine oxidase-catalyzed desymmetrization. J. Am. Chem. Soc. 134, 6467–6472 (2012).
Nielsen, L. P., Stevenson, C. P., Blackmond, D. G. & Jacobsen, E. N. Mechanistic investigation leads to a synthetic improvement in the hydrolytic kinetic resolution of terminal epoxides. J. Am. Chem. Soc. 126, 1360–1362 (2004).
van Dijk, L. et al. Mechanistic investigation of Rh(I)-catalysed asymmetric Suzuki–Miyaura coupling with racemic allyl halides. Nat. Catal. 4, 284–292 (2021).
Camasso, N. M. & Sanford, M. S. Design, synthesis, and carbon-heteroatom coupling reactions of organometallic nickel(IV) complexes. Science 347, 1218–1220 (2015).
Milo, A., Neel, A. J., Toste, F. D. & Sigman, M. S. A data-intensive approach to mechanistic elucidation applied to chiral anion catalysis. Science 347, 737–743 (2015).
Butcher, T. W. et al. Desymmetrization of difluoromethylene groups by C-F bond activation. Nature 583, 548–553 (2020).
Cho, E. J. et al. The palladium-catalyzed trifluoromethylation of aryl chlorides. Science 328, 1679–1681 (2010).
Hutchinson, G., Alamillo-Ferrer, C. & Bures, J. Mechanistically guided design of an efficient and enantioselective aminocatalytic alpha-chlorination of aldehydes. J. Am. Chem. Soc. 143, 6805–6809 (2021).
Schreyer, L. et al. Confined acids catalyze asymmetric single aldolizations of acetaldehyde enolates. Science 362, 216–219 (2018).
Peters, B. K. et al. Scalable and safe synthetic organic electroreduction inspired by Li-ion battery chemistry. Science 363, 838–845 (2019).
Michaelis, L. & Menten, M. L. Die Kinetik der Invertinwirkung. Biochem. Z. 49, 333–369 (1913).
Blackmond, D. G. Reaction progress kinetic analysis: a powerful methodology for mechanistic studies of complex catalytic reactions. Angew. Chem. Int. Ed. Engl. 44, 4302–4320 (2005).
Mathew, J. S. et al. Investigations of Pd-catalyzed ArX coupling reactions informed by reaction progress kinetic analysis. J. Org. Chem. 71, 4711–4722 (2006).
Bures, J. A simple graphical method to determine the order in catalyst. Angew. Chem. Int. Ed. Engl. 55, 2028–2031 (2016).
Burés, J. Variable time normalization analysis: general graphical elucidation of reaction orders from concentration profiles. Angew. Chem. Int. Ed. Engl. 55, 16084–16087 (2016).
Shi, Y., Prieto, P. L., Zepel, T., Grunert, S. & Hein, J. E. Automated experimentation powers data science in chemistry. Acc. Chem. Res. 54, 546–555 (2021).
Burger, B. et al. A mobile robotic chemist. Nature 583, 237–241 (2020).
Bedard, A. C. et al. Reconfigurable system for automated optimization of diverse chemical reactions. Science 361, 1220–1225 (2018).
Steiner, S. et al. Organic synthesis in a modular robotic system driven by a chemical programming language. Science 363, eaav2211 (2019).
Clauset, A., Shalizi, C. R. & Newman, M. E. J. Power-law distributions in empirical data. SIAM Rev. 51, 661–703 (2009).
Martinez-Carrion, A. et al. Kinetic treatments for catalyst activation and deactivation processes based on variable time normalization analysis. Angew. Chem. Int. Ed. Engl. 58, 10189–10193 (2019).
Bernacki, J. P. & Murphy, R. M. Model discrimination and mechanistic interpretation of kinetic data in protein aggregation studies. Biophys. J. 96, 2871–2887 (2009).
Pfluger, P. M. & Glorius, F. Molecular machine learning: the future of synthetic chemistry? Angew. Chem. Int. Ed. Engl. 59, 18860–18865 (2020).
Segler, M. H. S., Preuss, M. & Waller, M. P. Planning chemical syntheses with deep neural networks and symbolic AI. Nature 555, 604–610 (2018).
Raissi, M., Yazdani, A. & Karniadakis, G. E. Hidden fluid mechanics: learning velocity and pressure fields from flow visualizations. Science 367, 1026–1030 (2020).
Hermann, J., Schatzle, Z. & Noe, F. Deep-neural-network solution of the electronic Schrodinger equation. Nat. Chem. 12, 891–897 (2020).
Shields, B. J. et al. Bayesian reaction optimization as a tool for chemical synthesis. Nature 590, 89–96 (2021).
Tunyasuvunakool, K. et al. Highly accurate protein structure prediction for the human proteome. Nature 596, 590–596 (2021).
Jumper, J. et al. Highly accurate protein structure prediction with AlphaFold. Nature 596, 583–589 (2021).
Hueffel, J. A. et al. Accelerated dinuclear palladium catalyst identification through unsupervised machine learning. Science 374, 1134–1140 (2021).
Haitao, X., Junjie, W. & Lu, L. In Proc. 1st International Conference on E-Business Intelligence 303–309 (Atlantis Press, 2010).
Batista, G. E. A. P. A. et al. In Advances in Intelligent Data Analysis VI (eds Fazel Famili, A. et al.) 24–35 (Springer, 2005).
Wei, J.-M., Yuan, X.-J., Hu, Q.-H. & Wang, S.-Q. A novel measure for evaluating classifiers. Expert Syst. Appl. 37, 3799–3809 (2010).
Alberton, A. L., Schwaab, M., Schmal, M. & Pinto, J. C. Experimental errors in kinetic tests and its influence on the precision of estimated parameters. Part I—analysis of first-order reactions. Chem. Eng. J. 155, 816–823 (2009).
Pacheco, H., Thiengo, F., Schmal, M. & Pinto, J. C. A family of kinetic distributions for interpretation of experimental fluctuations in kinetic problems. Chem. Eng. J. 332, 303–311 (2018).
Storer, A. C., Darlison, M. G. & Cornish-Bowden, A. The nature of experimental error in enzyme kinetic measurments. Biochem. J 151, 361–367 (1975).
Valkó, É. & Turányi, T. In Lindner, E., Micheletti, A. & Nunes, C. (eds) Mathematical Modelling in Real Life Problems. Mathematics in Industry https://doi.org/10.1007/978-3-030-50388-8_3 (2020).
Thiel, V., Wannowius, K. J., Wolff, C., Thiele, C. M. & Plenio, H. Ring-closing metathesis reactions: interpretation of conversion-time data. Chem. Eur. J. 19, 16403–16414 (2013).
Joannou, M. V., Hoyt, J. M. & Chirik, P. J. Investigations into the mechanism of inter- and intramolecular iron-catalyzed [2 + 2] cycloaddition of alkenes. J. Am. Chem. Soc. 142, 5314–5330 (2020).
Knapp, S. M. M. et al. Mechanistic studies of alkene isomerization catalyzed by CCC-pincer complexes of iridium. Organometallics 33, 473–484 (2014).
Stroek, W., Keilwerth, M., Pividori, D. M., Meyer, K. & Albrecht, M. An iron-mesoionic carbene complex for catalytic intramolecular C-H amination utilizing organic azides. J. Am. Chem. Soc. 143, 20157–20165 (2021).
Lehnherr, D. et al. Discovery of a photoinduced dark catalytic cycle using in situ LED-NMR spectroscopy. J. Am. Chem. Soc. 140, 13843–13853 (2018).
Ludwig, J. R., Zimmerman, P. M., Gianino, J. B. & Schindler, C. S. Iron(III)-catalysed carbonyl-olefin metathesis. Nature 533, 374–379 (2016).
Albright, H. et al. Catalytic carbonyl-olefin metathesis of aliphatic ketones: iron(III) homo-dimers as Lewis acidic superelectrophiles. J. Am. Chem. Soc. 141, 1690–1700 (2019).
Janse van Rensburg, W., Steynberg, P. J., Meyer, W. H., Kirk, M. M. & Forman, G. S. DFT prediction and experimental observation of substrate-induced catalyst decomposition in ruthenium-catalyzed olefin metathesis. J. Am. Chem. Soc. 126, 14332–14333 (2004).
van der Eide, E. F. & Piers, W. E. Mechanistic insights into the ruthenium-catalysed diene ring-closing metathesis reaction. Nat. Chem. 2, 571–576 (2010).
Acknowledgements
We thank the European Research Council for an Advanced Grant (no. 833337 to I.L.) and Research IT for assistance given and use of the Computational Shared Facility at The University of Manchester. We thank H. Plenio, P. J. Chirick, A. R. Chianese, M. Albrecht and C. S. Schindler for providing the numerical experimental kinetic data used in this study.
Author information
Authors and Affiliations
Contributions
I.L. and J.B. conceived the project. J.B. selected the mechanisms with input from I.L. I.L. wrote the code, created the datasets and trained the models with input from J.B. I.L. and J.B. analysed data. I.L. and J.B. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Tiago Rodrigues and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Additional case study with experimental kinetic data.
Includes the reaction under study, the experimental kinetic data used as input for the AI-model and its output. Symbols correspond to substrate concentration. Red triangles: lowest catalyst loading; yellow squares: medium catalyst loading; blue circles: largest catalyst loading. Data from ref. 47.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Burés, J., Larrosa, I. Organic reaction mechanism classification using machine learning. Nature 613, 689–695 (2023). https://doi.org/10.1038/s41586-022-05639-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05639-4
This article is cited by
-
Intelligent synthesis of magnetic nanographenes via chemist-intuited atomic robotic probe
Nature Synthesis (2024)
-
Iterative design of training data to control intricate enzymatic reaction networks
Nature Communications (2024)
-
Machine learning classifies catalytic-reaction mechanisms
Nature (2023)
-
Automation, analytics and artificial intelligence for chemical synthesis
Nature Synthesis (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.