Abstract
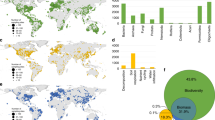
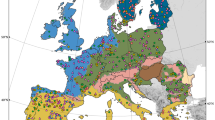
Soils are the foundation of all terrestrial ecosystems1. However, unlike for plants and animals, a global assessment of hotspots for soil nature conservation is still lacking2. This hampers our ability to establish nature conservation priorities for the multiple dimensions that support the soil system: from soil biodiversity to ecosystem services. Here, to identify global hotspots for soil nature conservation, we performed a global field survey that includes observations of biodiversity (archaea, bacteria, fungi, protists and invertebrates) and functions (critical for six ecosystem services) in 615 composite samples of topsoil from a standardized survey in all continents. We found that each of the different ecological dimensions of soils—that is, species richness (alpha diversity, measured as amplicon sequence variants), community dissimilarity and ecosystem services—peaked in contrasting regions of the planet, and were associated with different environmental factors. Temperate ecosystems showed the highest species richness, whereas community dissimilarity peaked in the tropics, and colder high-latitudinal ecosystems were identified as hotspots of ecosystem services. These findings highlight the complexities that are involved in simultaneously protecting multiple ecological dimensions of soil. We further show that most of these hotspots are not adequately covered by protected areas (more than 70%), and are vulnerable in the context of several scenarios of global change. Our global estimation of priorities for soil nature conservation highlights the importance of accounting for the multidimensionality of soil biodiversity and ecosystem services to conserve soils for future generations.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
All of the materials, raw data and protocols used in this Article are available upon request and without restriction, and all data are publicly available at https://doi.org/10.6084/m9.figshare.20221713.
References
Bardgett, R. D. & van der Putten, W. H. Belowground biodiversity and ecosystem functioning. Nature 515, 505–511 (2014).
Guerra, C. A. et al. Tracking, targeting, and conserving soil biodiversity. Science 371, 239–241 (2021).
Wall, D. H. et al. (eds) Soil Ecology and Ecosystem Services (Oxford University Press, 2012).
Jansson, J. K. & Hofmockel, K. S. Soil microbiomes and climate change. Nat. Rev. Microbiol. 18, 35–46 (2020).
de Vries, F. T. et al. Soil food web properties explain ecosystem services across European land use systems. Proc. Natl Acad. Sci. USA 110, 14296–14301 (2013).
Adhikari, K. & Hartemink, A. E. Linking soils to ecosystem services—a global review. Geoderma 262, 101–111 (2016).
Pereira, P., Bogunovic, I., Muñoz-Rojas, M. & Brevik, E. C. Soil ecosystem services, sustainability, valuation and management. Curr. Opin. Environ. Sci. Health 5, 7–13 (2018).
Wall, D. H., Nielsen, U. N. & Six, J. Soil biodiversity and human health. Nature 528, 69–76 (2015).
Delgado-Baquerizo, M. et al. The proportion of soil-borne pathogens increases with warming at the global scale. Nat. Clim. Chang. 10, 550–554 (2020).
Rillig, M. C. et al. The role of multiple global change factors in driving soil functions and microbial biodiversity. Science 366, 886–890 (2019).
Guerra, C. A. et al. Global vulnerability of soil ecosystems to erosion. Landsc. Ecol. 35, 823–842 (2020).
Geisen, S., Wall, D. H. & van der Putten, W. H. Challenges and opportunities for soil biodiversity in the Anthropocene. Curr. Biol. 29, R1036–R1044 (2019).
Jung, M. et al. Areas of global importance for conserving terrestrial biodiversity, carbon and water. Nat. Ecol. Evol. 5, 1499–1509 (2021).
Xu, H. et al. Ensuring effective implementation of the post-2020 global biodiversity targets. Nat. Ecol. Evol. 5, 411–418 (2021).
Díaz, S. et al. (eds). Summary for Policymakers of the Global Assessment Report on Biodiversity and Ecosystem Services of the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES, 2019); https://zenodo.org/record/3553579#.YyhIsXbMK70
Phillips, H. R. P. et al. Global distribution of earthworm diversity. Science 366, 480–485 (2019).
van den Hoogen, J. et al. Soil nematode abundance and functional group composition at a global scale. Nature 572, 194–198 (2019).
Delgado-baquerizo, M. et al. A global atlas of the dominant bacteria found in soil. Science 325, 320–325 (2018).
Tedersoo, L. et al. Global diversity and geography of soil fungi. Science 346, 1256688 (2014).
Xu, X., Thornton, P. E. & Post, W. M. A global analysis of soil microbial biomass carbon, nitrogen and phosphorus in terrestrial ecosystems: global soil microbial biomass C, N and P. Glob. Ecol. Biogeogr. 22, 737–749 (2013).
Djukic, I. et al. Early stage litter decomposition across biomes. Sci. Total Environ. 628–629, 1369–1394 (2018).
Guerra, C. A. et al. Global projections of the soil microbiome in the Anthropocene. Glob. Ecol. Biogeogr. 30, 987–999 (2021).
Cameron, E. K. et al. Global mismatches in aboveground and belowground biodiversity. Conserv. Biol. 33, 1187–1192 (2019).
El Moujahid, L. et al. Effect of plant diversity on the diversity of soil organic compounds. PLoS One 12, e0170494 (2017).
Guerra, C. A. et al. Blind spots in global soil biodiversity and ecosystem function research. Nat. Commun. 11, 3870 (2020).
Fierer, N. & Jackson, R. B. The diversity and biogeography of soil bacterial communities. Proc. Natl Acad. Sci. USA 103, 626–631 (2006).
Tedersoo, L. et al. Regional-scale in-depth analysis of soil fungal diversity reveals strong pH and plant species effects in Northern Europe. Front. Microbiol. 11, 1953 (2020).
Popp, A. et al. Land-use futures in the shared socio-economic pathways. Glob. Environ. Change 42, 331–345 (2017).
Dornelas, M. et al. Assemblage time series reveal biodiversity change but not systematic loss. Science 344, 296–299 (2014).
Egoh, B., Reyers, B., Rouget, M., Bode, M. & Richardson, D. M. Spatial congruence between biodiversity and ecosystem services in South Africa. Biol. Conserv. 142, 553–562 (2009).
Jürgens, N. et al. The BIOTA Biodiversity Observatories in Africa—a standardized framework for large-scale environmental monitoring. Environ. Monit. Assess. 184, 655–678 (2012).
Wyborn, C. & Evans, M. C. Conservation needs to break free from global priority mapping. Nat. Ecol. Evol. 5, 1322–1324 (2021).
Hautier, Y. et al. Local loss and spatial homogenization of plant diversity reduce ecosystem multifunctionality. Nat. Ecol. Evol. 2, 50–56 (2018).
Zhou, Z., Wang, C. & Luo, Y. Meta-analysis of the impacts of global change factors on soil microbial diversity and functionality. Nat. Commun. 11, 3072 (2020).
Eisenhauer, N., Schulz, W., Scheu, S. & Jousset, A. Niche dimensionality links biodiversity and invasibility of microbial communities. Funct. Ecol. 27, 282–288 (2013).
Wagg, C., Bender, S. F., Widmer, F. & van der Heijden, M. G. A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl Acad. Sci. USA 111, 5266–5270 (2014).
Haines-Young, R. H. & Potschin, M. B. in Ecosystems Ecology: A New Synthesis (eds Raffaelli, D. G. & Frid, C. L. J.) Ch. 6 (2012).
Smith, L. C. et al. Large‐scale drivers of relationships between soil microbial properties and organic carbon across Europe. Glob. Ecol. Biogeogr. 30, 2070–2083 (2021).
Keesstra, S. et al. The superior effect of nature based solutions in land management for enhancing ecosystem services. Sci. Total Environ. 610-611, 997–1009 (2018).
Le Provost, G. et al. Contrasting responses of above- and belowground diversity to multiple components of land-use intensity. Nat. Commun. 12, 3918 (2021).
Tanneberger, F. et al. The power of nature‐based solutions: how peatlands can help us to achieve key EU sustainability objectives. Adv. Sustain. Syst. 5, 2000146 (2021).
Johnston, A. et al. Observed and predicted effects of climate change on species abundance in protected areas. Nat. Clim. Chang. 3, 1055–1061 (2013).
Hannah, L. et al. Protected area needs in a changing climate. Front. Ecol. Environ. 5, 131–138 (2007).
Gallardo, B. et al. Protected areas offer refuge from invasive species spreading under climate change. Glob. Chang. Biol. 23, 5331–5343 (2017).
O’Neill, B. C. et al. The roads ahead: narratives for shared socioeconomic pathways describing world futures in the 21st century. Glob. Environ. Change 42, 169–180 (2017).
Fedele, G., Donatti, C. I., Bornacelly, I. & Hole, D. G. Nature-dependent people: mapping human direct use of nature for basic needs across the tropics. Glob. Environ. Change 71, 102368 (2021).
Visconti, P. et al. Protected area targets post-2020. Science 364, 239–241 (2019).
Allan, J. R. et al. The minimum land area requiring conservation attention to safeguard biodiversity. Science 376, 1094–1101 (2022).
Maestre, F. T. et al. Plant species richness and ecosystem multifunctionality in global drylands. Science 335, 214–218 (2012).
Delgado-Baquerizo, M. et al. Changes in belowground biodiversity during ecosystem development. Proc. Natl Acad. Sci. USA. 116, 6891–6896 (2019).
Mace, G. M. Whose conservation? Science 345, 1558–1560 (2014).
Amaral-Zettler, L. A., McCliment, E. A., Ducklow, H. W. & Huse, S. M. A method for studying protistan diversity using massively parallel sequencing of V9 hypervariable regions of small-subunit ribosomal RNA genes. PLoS One 4, e6372 (2009).
Stoeck, T. et al. Multiple marker parallel tag environmental DNA sequencing reveals a highly complex eukaryotic community in marine anoxic water. Mol. Ecol. 19, 21–31 (2010).
Ramirez, K. S. et al. Biogeographic patterns in below-ground diversity in New York City’s Central Park are similar to those observed globally. Proc. Biol. Sci. 281, 20141988 (2014).
Caporaso, J. G. et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336 (2010).
Edgar, R. C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26, 2460–2461 (2010).
Edgar, R. C. & Flyvbjerg, H. Error filtering, pair assembly and error correction for next-generation sequencing reads. Bioinformatics 31, 3476–3482 (2015).
Edgar, R. C. UNOISE2: improved error-correction for Illumina 16S and ITS amplicon sequencing. Preprint at bioRxiv https://doi.org/10.1101/081257 (2016).
Tedersoo, L. et al. Towards understanding diversity, endemicity and global change vulnerability of soil fungi. Preprint at bioRxiv https://doi.org/10.1101/2022.03.17.484796 (2022).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596 (2013).
Delgado-Baquerizo, M. et al. Global homogenization of the structure and function in the soil microbiome of urban greenspaces. Sci. Adv. 7, eabg5809 (2021).
Phillips, H. R. P., Heintz-Buschart, A. & Eisenhauer, N. Putting soil invertebrate diversity on the map. Mol. Ecol. 29, 655–657 (2020).
Xiong, W. et al. A global overview of the trophic structure within microbiomes across ecosystems. Environ. Int. 151, 106438 (2021).
Drummond, A. J. et al. Evaluating a multigene environmental DNA approach for biodiversity assessment. Gigascience 4, 46 (2015).
Oliverio, A. M., Gan, H., Wickings, K. & Fierer, N. A DNA metabarcoding approach to characterize soil arthropod communities. Soil Biol. Biochem. 125, 37–43 (2018).
Horton, D. J., Kershner, M. W. & Blackwood, C. B. Suitability of PCR primers for characterizing invertebrate communities from soil and leaf litter targeting metazoan 18S ribosomal or cytochrome oxidase I (COI) genes. Eur. J. Soil Biol. 80, 43–48 (2017).
Delgado-Baquerizo, M. et al. Multiple elements of soil biodiversity drive ecosystem functions across biomes. Nat. Ecol. Evol. 4, 210–220 (2020).
Carter, M. R. & Gregorich, E. G. (eds) Soil Sampling and Methods of Analysis (CRC Press, 2007).
Sparks, D. L. et al. (eds) Methods of Soil Analysis, Part 3: Chemical Methods (Wiley, 2020).
Nguyen, N. H. et al. FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 20, 241–248 (2016).
Bell, C. W. et al. High-throughput fluorometric measurement of potential soil extracellular enzyme activities. J. Vis. Exp. 81, e50961 (2013).
Wang, L. et al. Diversifying livestock promotes multidiversity and multifunctionality in managed grasslands. Proc. Natl Acad. Sci. USA. 116, 6187–6192 (2019).
Durán, J., Delgado-Baquerizo, M., Rodríguez, A., Covelo, F. & Gallardo, A. Ionic exchange membranes (IEMs): a good indicator of soil inorganic N production. Soil Biol. Biochem. 57, 964–968 (2013).
Breiman, L. Random forests. Mach. Learn. 45, 5–32 (2001).
Friedman, J. H. Greedy function approximation: a gradient boosting machine. Ann. Stat. 29, 1189–1232 (2001).
Sharma, N. XGBoost. The Extreme Gradient Boosting for Mining Applications (GRIN Verlag, 2018).
Chen, T. & Guestrin, C. XGBoost: a scalable tree boosting system. In Proc. 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining 785–794 (Association for Computing Machinery, 2016).
Wilson. ParBayesianOptimization: Parallel Bayesian Optimization of Hyperparameters. R version 1 https://CRAN.R-project.org/package=ParBayesianOptimization (2021).
Hastie, T., Friedman, J. & Tibshirani, R. The Elements of Statistical Learning (Springer, 2001).
Jackson, D. A. & Chen, Y. Robust principal component analysis and outlier detection with ecological data. Environmetrics 15, 129–139 (2004).
Breiman, L. Bagging predictors. Mach. Learn. 24, 123–140 (1996).
Breiman, L., Friedman, J., Stone, C. J. & Olshen, R. A. Classification and Regression Trees (Routledge, 1984).
Ord, J. K. & Getis, A. Local spatial autocorrelation statistics: distributional issues and an application. Geogr. Anal. 27, 286–306 (2010).
Getis, A. & Ord, J. K. The analysis of spatial association by use of distance statistics. Geogr. Anal. 24, 189–206 (2010).
Prasannakumar, V., Vijith, H., Charutha, R. & Geetha, N. Spatio-temporal clustering of road accidents: GIS based analysis and assessment. Procedia Soc. Behav. Sci. 21, 317–325 (2011).
Lin, G. Comparing spatial clustering tests based on rare to common spatial events. Comput. Environ. Urban Syst. 28, 691–699 (2004).
Araújo, M. B. et al. Standards for distribution models in biodiversity assessments. Sci. Adv. 5, eaat4858 (2019).
Rousseeuw, P. J. & van Zomeren, B. C. Unmasking multivariate outliers and leverage points. J. Am. Stat. Assoc. 85, 633–639 (1990).
Hempel, S., Frieler, K., Warszawski, L., Schewe, J. & Piontek, F. A trend-preserving bias correction—the ISI-MIP approach. Earth Syst. Dyn. 4, 219–236 (2013).
Lawrence, D. M. et al. The Land Use Model Intercomparison Project (LUMIP) contribution to CMIP6: rationale and experimental design. Geosci. Model Dev. 9, 2973–2998 (2016).
Kim, H. et al. A protocol for an intercomparison of biodiversity and ecosystem services models using harmonized land-use and climate scenarios. Geosci. Model Dev. 11, 4537–4562 (2018).
Dufresne, J.-L. et al. Climate change projections using the IPSL-CM5 Earth System Model: from CMIP3 to CMIP5. Clim. Dyn. 40, 2123–2165 (2013).
Hurtt, G. C. et al. Harmonization of land-use scenarios for the period 1500–2100: 600 years of global gridded annual land-use transitions, wood harvest, and resulting secondary lands. Clim. Change 109, 117 (2011).
Hurtt, G. C. et al. Harmonization of global land use change and management for the period 850–2100 (LUH2) for CMIP6. Geosci. Model Dev. 13, 5425–5464 (2020).
Riahi, K. et al. The Shared Socioeconomic Pathways and their energy, land use, and greenhouse gas emissions implications: an overview. Glob. Environ. Change 42, 153–168 (2017).
O’Neill, B. C. et al. A new scenario framework for climate change research: the concept of shared socioeconomic pathways. Clim. Change 122, 387–400 (2014).
Newbold, T. et al. Global effects of land use on local terrestrial biodiversity. Nature 520, 45–50 (2015).
Powers, R. P. & Jetz, W. Global habitat loss and extinction risk of terrestrial vertebrates under future land-use-change scenarios. Nat. Clim. Chang. 9, 323–329 (2019).
Acknowledgements
We thank all of the researchers who were involved in the collection of field data. This project received funding from the British Ecological Society (agreement LRA17\1193; MUSGONET). C.A.G. and N.E. were funded by DFG–FZT 118, 202548816; C.A.G. was supported by FCT-PTDC/BIA-CBI/2340/2020; M.D.-B. was supported by RYC2018-025483-I, PID2020-115813RA-I00\MCIN/AEI/10.13039/501100011033 and P20_00879. M.A.M.-M. and S.A. were funded by FONDECYT 1181034 and ANID-PIA-Anillo INACH ACT192057. J.D. and A.R. acknowledge support from IF/00950/2014, 2020.03670.CEECIND, SFRH/BDP/108913/2015 and UIDB/04004/2020. Y.-R.L. was supported by 2662019PY010 from the FRFCU. L.T. was supported by the ESF grant PRG632. F.B. and J.L.M. were supported by i-LINK+2018 (LINKA20069) funded by CSIC. C.T.-D. was supported by the Grupo de Biodibersidad & Cambio Global UBB–GI 170509/EF. C.P. was supported by the EU H2020 grant agreement 101000224. H.C. was supported by NSFC32101335, FRFCU2412021QD014 and CPSF2021M690589. J.P.V. was supported by DST (DST/INT/SL/P-31/2021) SERB (EEQ/2021/001083) and BHU-IoE (6031).
Author information
Authors and Affiliations
Contributions
C.A.G. and M.D.-B. developed the original idea of the analyses presented in the manuscript. M.D.-B. designed the field study and wrote the grant that funded the work. Field data were collected by M.B., S.A., F.D.A., A.R.B., J.L.B.-P., A.d.l.R., J.D., T.G., J.G.I., Y.-R.L., T.P.M., S.M., M.A.M.-M., A.M., T.U.N., G.F.P.-B., C.P., J.P.V., A. Rey, A. Rodríguez, A.L.T., C.T.-D., P.T., L.W., Jianyong Wang, E.Z., X.Z., X.-Q. Z. and M.D.-B. Laboratory analyses were done by M.D.-B., H.C., F.B., J.L.M., S.P. and L.T. Statistical analyses, mapping and ecological modelling were done by C.A.G., M.D.-B. and M.B. Bioinformatic analyses were done by B.K.S. and Juntao Wang. The manuscript was written by C.A.G. and M.D-B. and edited by N.E. and D.J.E., with contributions from all co-authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Peter de Ruiter, Ruhollah Taghizadeh and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
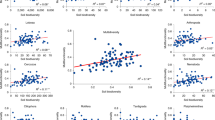
Extended Data Fig. 1 Results of the random forest analysis to identify the main environmental factors associated with soil biodiversity and ecosystem services.
Random forest analyses were done using the rfPermute function of the R package with the same name. MSE, mean square error.
Extended Data Fig. 2 Spearman correlations between environmental factors and soil biodiversity and ecosystem services.
N in Supplementary Table 1.
Extended Data Fig. 3 Spearman correlations between soil biodiversity and ecosystem services.
Total n-values in Supplementary Table 1.
Extended Data Fig. 4 Hotspot and coldspot maps for alpha diversity (left) and community dissimilarity (right).
The Getis-Ord Gi* statistic was calculated for each location (0.25x0.25 deg pixel size) in the dataset 1–3. The resulting z-scores were used to estimate if a given location has statistically high or low values and if these values are spatially clustered. This is done by assessing each location within the context of neighbouring locations. Statistically significant positive z-scores indicate clustering of high values (hotspot) and statistically significant negative z-scores the clustering of low values (coldspot). Values are plotted for both positive (hotspots) and negative (coldspots) 99%, 95%, and 90% confidence levels.
Extended Data Fig. 5 Hotspot and coldspot maps for ecosystem services: soil carbon, fertility, organic matter decomposition, pest control, mutualism and water retention.
The Getis-Ord Gi* statistic was calculated for each location (0.25x0.25 deg pixel size) in the dataset 1–3. The resulting z-scores were used to estimate if a given location has statistically high or low values and if these values are spatially clustered. This is done by assessing each location within the context of neighbouring locations. Statistically significant positive z-scores indicate clustering of high values (hotspot) and statistically significant negative z-scores the clustering of low values (coldspot). Values are plotted for both positive (hotspots) and negative (coldspots) 99%, 95%, and 90% confidence levels.
Supplementary information
Supplementary Information
This file includes all supplementary figures (S1–S17) and supplementary tables (S1–S15) that support our paper.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guerra, C.A., Berdugo, M., Eldridge, D.J. et al. Global hotspots for soil nature conservation. Nature 610, 693–698 (2022). https://doi.org/10.1038/s41586-022-05292-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05292-x
This article is cited by
-
Biotic homogenization, lower soil fungal diversity and fewer rare taxa in arable soils across Europe
Nature Communications (2024)
-
Multiple invasion routes have led to the pervasive introduction of earthworms in North America
Nature Ecology & Evolution (2024)
-
Soil microbial diversity plays an important role in resisting and restoring degraded ecosystems
Plant and Soil (2024)
-
The diversity and abundance of soil macrofauna under different agroforestry practices in the drylands of southern Ethiopia
Agroforestry Systems (2024)
-
Effects of protected areas on soil nematode communities in forests of the North of Portugal
Biodiversity and Conservation (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.