Abstract
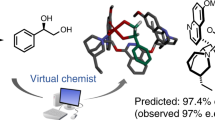
Research in the field of asymmetric catalysis over the past half century has resulted in landmark advances, enabling the efficient synthesis of chiral building blocks, pharmaceuticals and natural products1,2,3. A small number of asymmetric catalytic reactions have been identified that display high selectivity across a broad scope of substrates; not coincidentally, these are the reactions that have the greatest impact on how enantioenriched compounds are synthesized4,5,6,7,8. We postulate that substrate generality in asymmetric catalysis is rare not simply because it is intrinsically difficult to achieve, but also because of the way chiral catalysts are identified and optimized9. Typical discovery campaigns rely on a single model substrate, and thus select for high performance in a narrow region of chemical space. Here we put forth a practical approach for using multiple model substrates to select simultaneously for both enantioselectivity and generality in asymmetric catalytic reactions from the outset10,11. Multisubstrate screening is achieved by conducting high-throughput chiral analyses by supercritical fluid chromatography–mass spectrometry with pooled samples. When applied to Pictet–Spengler reactions, the multisubstrate screening approach revealed a promising and unexpected lead for the general enantioselective catalysis of this important transformation, which even displayed high enantioselectivity for substrate combinations outside of the screening set.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data are available in the main text or the supplementary materials.
Code availability
The Python library used for SFC–MS peak deconvolution and analysis is available on Github under the GPL 3.0 license (https://github.com/corinwagen/chromatics).
References
Knowles, W. S., Sabacky, M. J. & Büthe, H. Catalytic asymmetric hydrogenation employing a soluble, optically active, rhodium complex. Chem. Commun. (London) 1445–1446 (1968).
Horner, L., Siegel, H. & Büthe, H. Asymmetric catalytic hydrogenation with an optically active phosphine–rhodium complex in homogeneous solution. Angew. Chem. Int. Ed. 7, 942 (1968).
Jacobsen, E. N., Pfaltz, A. & Yamamoto, H. (eds) Comprehensive Asymmetric Catalysis Vols. 1–3 (Springer, 1999).
Katsuki, T. & Sharpless, K. B. The first practical method for asymmetric epoxidation. J. Am. Chem. Soc. 102, 5974–5976 (1980).
Jacobsen, E. N., Marko, I., Mungall, W. S., Schroeder, G. & Sharpless, K. B. Asymmetric dihydroxylation via ligand-accelerated catalysis. J. Am. Chem. Soc. 110, 1968–1970 (1988).
Noyori, R. et al. Asymmetric hydrogenation of β-keto carboxylic esters. A practical, purely chemical access to β-hydroxy esters in high enantiomeric purity. J. Am. Chem. Soc. 109, 5856–5858 (1989).
Hirao, A., Itsuno, S., Nakahama, S. & Yamazaki, N. Asymmetric reduction of aromatic ketones with chiral alkoxy-amineborane complexes. J. Chem. Soc. Chem. Commun. 315–317 (1981).
Tokunaga, M., Larrow, J. F., Kakiuchi, F. & Jacobsen, E. N. Asymmetric catalysis with water: efficient kinetic resolution of terminal epoxides by means of catalytic hydrolysis. Science 277, 936–938 (1997).
Schmidt-Dannert, C. & Arnold, F. H. Directed evolution of industrial enzymes. Trends Biotechnol. 17, 135–136 (1999).
Gao, X. & Kagan, H. B. One-pot multi-substrate screening in asymmetric catalysis. Chirality 10, 120–124 (1998).
Satyanarayana, T. & Kagan, H. B. The multi-substrate screening of asymmetric catalysis. Adv. Synth. Catal. 347, 737–748 (2005).
Kim, H. et al. A multi-substrate screening approach for the identification of a broadly applicable Diels–Alder catalyst. Nat. Commun. 10, 770 (2019).
Prieto Kullmer, C. N. et al. Accelerating reaction generality and mechanistic insight through additive mapping. Science 376, 532–539 (2022).
You, L., Zha, D. & Ansyln, E. V. Recent advances in supramolecular analytical chemistry using optical sensing. Chem. Rev. 115, 7840–7892 (2015).
Herrera, B. T., Pilicer, S. L., Ansyln, E. V., Joyce, L. A. & Wolf, C. Optical analysis of reaction yield and enantiomeric excess. A new paradigm ready for prime time. J. Am. Chem. Soc. 140, 10385–10401 (2018).
Jang, S. & Kim, H. Direct chiral 19F NMR analysis of fluorine-containing analytes and its application to simultaneous chiral analysis. Org. Lett. 22, 7804–7808 (2020).
Feagin, T. A., Olsen, D. P. V., Headman, Z. C. & Heemstra, J. M. High-throughput enantiopurity analysis using enantiomeric DNA-based sensors. J. Am. Chem. Soc. 137, 4198–4206 (2015).
Guo, J., Wu, J., Siuzdak, G. & Finn, M. G. Measurement of enantiomeric excess by kinetic resolution and mass spectrometry. Angew. Chem. Int. Ed. 38, 1755–1758 (1999).
Reetz, M. T., Becker, M. H., Klein, H.-W. & Stöckigt, D. A method for high-throughput screening of enantioselective catalysts. Angew. Chem. Int. Ed. 38, 1758–1761 (1999).
Abato, P. & Seto, C. T. EMDee: an enzymatic method for determining enantiomeric excess. J. Am. Chem. Soc. 123, 9206–9207 (2001).
Zhao, Y., Woo, G., Thomas, S., Semin, D. & Sandra, P. Rapid method development for chiral separation in drug discovery using sample pooling and supercritical fluid chromatography–mass spectrometry. J. Chrom. A 1003, 157–166 (2003).
Barhate, C. L. et al. Ultrafast chiral separations for high throughput enantiopurity analysis. Chem. Commun. 53, 509–512 (2017).
Shen, J., Ikai, T. & Okamoto, Y. Synthesis and application of immobilized polysaccharide-based chiral stationary phases for enantioseparation by high-performance liquid chromatography. J. Chromatogr. A 1363, 51–61 (2014).
Korch, K. M. et al. Selected ion monitoring using low-cost mass spectrum detectors provides a rapid, general, and accurate method for enantiomeric excess determination in high-throughput experimentation. ACS Catal. 12, 6737–6745 (2022).
Payne, C. & Kass, S. R. How reliable are enantiomeric excess measurements obtained by chiral HPLC? ChemistrySelect 5, 1810–1817 (2020).
Annesley, T. Ion suppression in mass spectrometry. Clin. Chem. 49, 1041–1044 (2003).
George, R. et al. Enhancement and suppression of ionization in drug analysis using HPLC-MS/MS in support of therapeutic drug monitoring: a review of current knowledge of its minimization and assessment. Ther. Drug. Monit. 40, 1–8 (2018).
Cox, E. D. & Cook, J. M. The Pictet-Spengler condensation: a new direction for an old reaction. Chem. Rev. 95, 1797–1842 (1995).
Stöckigt, J., Antonchick, A. P., Wu, F. & Walmann, H. The Pictet–Spengler reaction in nature and in organic chemistry. Angew. Chem. Int. Ed. 50, 8538–8564 (2011).
Taylor, M. S. & Jacobsen, E. N. Highly enantioselective catalytic acyl-Pictet–Spengler reactions. J. Am. Chem. Soc. 126, 10558–10559 (2004).
Seayad, J., Seayad, A. M. & List, B. Catalytic asymmetric Pictet–Spengler reaction. J. Am. Chem. Soc. 128, 1086–1087 (2006).
Wanner, M. J., van der Haas, R. N. S., de Cuba, K. R., van Maarseveen, J. H. & Hiemstra, H. Catalytic asymmetric Pictet–Spengler reactions via sulfenyliminium ions. Angew. Chem. Int. Ed. 46, 7485–7487 (2007).
Raheem, I. T., Thiara, P. V., Peterson, E. A. & Jacobsen, E. N. Enantioselective Pictet–Spengler-type cyclizations of hydroxylactams: H-bond donor catalysis by anion binding. J. Am. Chem. Soc. 129, 13404–13405 (2007).
Sewgobind, N. V. et al. Enantioselective BINOL-phosphoric acid catalyzed Pictet−Spengler reactions of N-benzyltryptamine. J. Org. Chem. 73, 6405–6408 (2008).
Huang, D., Xu, F., Lin, X. & Wang, Y. Highly enantioselective Pictet–Spengler reaction catalyzed by SPINOL‐phosphoric acids. Chem. Eur. J. 18, 3148–3152 (2012).
Mittal, N., Sun, D. X. & Seidel, D. Conjugate-base-stabilized Brønsted acids: catalytic enantioselective Pictet–Spengler reactions with unmodified tryptamine. Org. Lett. 16, 1012–1015 (2014).
Qi, L., Hou, H., Ling, F. & Zhong, W. The cinchona alkaloid squaramide catalyzed asymmetric Pictet–Spengler reaction and related theoretical studies. Org. Biomol. Chem. 16, 566–574 (2018).
Glinsky-Olivier, N., Yang, S., Retailleau, P., Gandon, V. & Guinchard, X. Enantioselective gold-catalyzed Pictet–Spengler reaction. Org. Lett. 21, 9446–9451 (2019).
Kondo, M. et al. Practical stereoselective synthesis of C3‐spirooxindole‐ and C2‐spiropseudoindoxyl‐pyrrolidines via organocatalyzed Pictet‐Spengler reaction/oxidative rearrangement sequence. Adv. Synth. Catal. 363, 2648–2663 (2021).
Lynch-Colameta, T., Greta, S. & Snyder, S. A. Synthesis of aza-quaternary centers via Pictet–Spengler reactions of ketonitrones. Chem. Sci. 12, 6181–6187 (2021).
Muratore, M. E. et al. Enantioselective Brønsted acid-catalyzed N-acyliminium cyclization cascades. J. Am. Chem. Soc. 131, 10796–10797 (2009).
Klausen, R. S. & Jacobsen, E. N. Weak Brønsted acid-thiourea co-catalysis: enantioselective, catalytic protio-Pictet-Spengler reactions. Org. Lett. 11, 887–890 (2009).
Nakamura, S., Matsuda, Y., Takehara, T. & Suzuki, T. Enantioselective Pictet−Spengler reaction of acyclic α‐ketoesters using chiral imidazoline-phosphoric acid catalysts. Org. Lett. 24, 1072–1076 (2022).
Chan, Y.-C., Sak, M. H., Frank, S. A. & Miller, S. J. Tunable and cooperative catalysis for enantioselective Pictet-Spengler reaction with varied nitrogen-containing heterocyclic carboxaldehydes. Angew. Chem. Int. Ed. 60, 24573–24581 (2021).
McInnes, L., Healy, J. & Melville, J. UMAP: uniform manifold approximation and projection for dimension reduction. Preprint at https://arxiv.org/abs/1802.03426 (2018).
Acknowledgements
We thank J. Gair, R. Klausen, R. Liu, A. Makarov, L. Nogle and E. Regalado for helpful discussions. This work was supported by a NIH grant GM043214 (E.N.J.), Dean’s Competitive Fund, Harvard University (E.N.J.) and NSF predoctoral fellowship DGE1745303 (C.C.W.).
Author information
Authors and Affiliations
Contributions
C.C.W., E.E.K. and E.N.J. conceived the work, C.C.W., S.E.M. and E.E.K. designed and validated the analytical method, C.C.W. and S.E.M. conducted the high-throughput screens. E.E.K. and E.N.J. directed the research, and all authors wrote and edited the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Andrea Gargano and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Supplementary Sections 1–6, including general information, details regarding analytical method development and validation text, application to the Pictet–Spengler reaction, References, NMR spectra data and further Supplementary Data. These sections include Supplementary Figs. 1–47 and Tables 1–10.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wagen, C.C., McMinn, S.E., Kwan, E.E. et al. Screening for generality in asymmetric catalysis. Nature 610, 680–686 (2022). https://doi.org/10.1038/s41586-022-05263-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05263-2
This article is cited by
-
Accelerated discovery of molecular nanojunction photocatalysts for hydrogen evolution by using automated screening and flow synthesis
Nature Synthesis (2024)
-
Identifying general reaction conditions by bandit optimization
Nature (2024)
-
‘Bandit’ algorithms help chemists to discover generally applicable conditions for reactions
Nature (2024)
-
All-round catalytic and atroposelective strategy via dynamic kinetic resolution for N-/2-/3-arylindoles
Nature Communications (2023)
-
Rapid planning and analysis of high-throughput experiment arrays for reaction discovery
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.