Abstract
Molecular studies suggest that the origin of jawed vertebrates was no later than the Late Ordovician period (around 450 million years ago (Ma))1,2. Together with disarticulated micro-remains of putative chondrichthyans from the Ordovician and early Silurian period3,4,5,6,7,8, these analyses suggest an evolutionary proliferation of jawed vertebrates before, and immediately after, the end-Ordovician mass extinction. However, until now, the earliest complete fossils of jawed fishes for which a detailed reconstruction of their morphology was possible came from late Silurian assemblages (about 425 Ma)9,10,11,12,13. The dearth of articulated, whole-body fossils from before the late Silurian has long rendered the earliest history of jawed vertebrates obscure. Here we report a newly discovered Konservat-Lagerstätte, which is marked by the presence of diverse, well-preserved jawed fishes with complete bodies, from the early Silurian (Telychian age, around 436 Ma) of Chongqing, South China. The dominant species, a ‘placoderm’ or jawed stem gnathostome, which we name Xiushanosteus mirabilis gen. et sp. nov., combines characters from major placoderm subgroups14,15,16,17 and foreshadows the transformation of the skull roof pattern from the placoderm to the osteichthyan condition10. The chondrichthyan Shenacanthus vermiformis gen. et sp. nov. exhibits extensive thoracic armour plates that were previously unknown in this lineage, and include a large median dorsal plate as in placoderms14,15,16, combined with a conventional chondrichthyan bauplan18,19. Together, these species reveal a previously unseen diversification of jawed vertebrates in the early Silurian, and provide detailed insights into the whole-body morphology of the jawed vertebrates of this period.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data analysed in this paper, including the phylogenetic datasets, are available as part of the Article, Extended Data Figs. 1–10 or the Supplementary Information. Supplementary Data 1 and 5 are available at Figshare (https://doi.org/10.6084/m9.figshare.20317233.v1). The nomenclature described in this publication has been registered at ZooBank (LSID: urn:lsid:zoobank.org:pub: A85E3402-5F4D-4694-8BB9-90D2D87BB1D7).
References
Irisarri, I. et al. Phylotranscriptomic consolidation of the jawed vertebrate timetree. Nat. Ecol. Evol. 1, 1370–1378 (2017).
Simakov, O. et al. Deeply conserved synteny resolves early events in vertebrate evolution. Nat. Ecol. Evol. 4, 820–830 (2020).
Sansom, I. J., Davies, N. S., Coates, M. I., Nicoll, R. S. & Ritchie, A. Chondrichthyan-like scales from the Middle Ordovician of Australia. Palaeontology 55, 243–247 (2012).
Andreev, P. S. et al. Upper Ordovician chondrichthyan-like scales from North America. Palaeontology 58, 691–704 (2015).
Andreev, P. et al. The systematics of the Mongolepidida (Chondrichthyes) and the Ordovician origins of the clade. PeerJ 4, e1850 (2016).
Sansom, I. J., Wang, N.-Z. & Smith, M. The histology and affinities of sinacanthid fishes: primitive gnathostomes from the Silurian of China. Zool. J. Linn. Soc. 144, 379–386 (2005).
Zhu, M. Early Silurian sinacanths (Chondrichthyes) from China. Palaeontology 41, 157–171 (1998).
Andreev, P. S. et al. Elegestolepis and its kin, the earliest monodontode chondrichthyans. J. Vert. Paleontol. 37, e1245664 (2017).
Zhu, M. et al. The oldest articulated osteichthyan reveals mosaic gnathostome characters. Nature 458, 469–474 (2009).
Zhu, M. et al. A Silurian placoderm with osteichthyan-like marginal jaw bones. Nature 502, 188–193 (2013).
Choo, B., Zhu, M., Zhao, W., Jia, L. & Zhu, Y. The largest Silurian vertebrate and its palaeoecological implications. Sci. Rep. 4, 5242 (2014).
Li, Q. et al. A new Silurian fish close to the common ancestor of modern gnathostomes. Curr. Biol. 31, 3613–3620 (2021).
Burrow, C. J. & Rudkin, D. Oldest near-complete acanthodian: the first vertebrate from the Silurian Bertie Formation Konservat-Lagerstätte, Ontario. PLoS ONE 9, e104171 (2014).
Goujet, D. F. in Major Events in Early Vertebrate Evolution: Palaeontology, Phylogeny, Genetics and Development (ed. Ahlberg, P. E.) 209–222 (Taylor & Francis, 2001).
Goujet, D. F. & Young, G. C. in Recent Advances in the Origin and Early Radiation of Vertebrates (eds Arratia, G. et al.) 109–126 (Verlag Dr. Friedrich Pfeil, 2004).
Young, G. C. Placoderms (armored fish): dominant vertebrates of the Devonian period. Annu. Rev. Earth Planet. Sci. 38, 523–550 (2010).
Dupret, V., Sanchez, S., Goujet, D., Tafforeau, P. & Ahlberg, P. E. A primitive placoderm sheds light on the origin of the jawed vertebrate face. Nature 507, 500–503 (2014).
Coates, M. I. et al. An early chondrichthyan and the evolutionary assembly of a shark body plan. Proc. R. Soc. B 285, 20172418 (2018).
Miller, R. F., Cloutier, R. & Turner, S. The oldest articulated chondrichthyan from the Early Devonian period. Nature 425, 501–504 (2003).
Zhu, M. et al. A Silurian maxillate placoderm illuminates jaw evolution. Science 354, 334–336 (2016).
Zhu, Y.-A., Lu, J. & Zhu, M. Reappraisal of the Silurian placoderm Silurolepis and insights into the dermal neck joint evolution. R. Soc. Open Sci. 6, 191181 (2019).
Liu, Y.-H. in Early Vertebrates and Related Problems of Evolutionary Biology (eds Chang, M.-M. et al.) 139–177 (Science Press, 1991).
Dennis-Bryan, K. A new species of eastmanosteid arthrodire (Pisces: Placodermi) from Gogo, Western Australia. Zool. J. Linn. Soc. 90, 1–64 (1987).
Gross, W. Lunaspis broilii und Lunaspis heroldi aus dem Hunsrückschiefer (Unterdevon, Rheinland). Notizbl. Hess. Landesamt. Bodenforsch. Wiesb. 89, 17–43 (1961).
Dearden, R. P., Stockey, C. & Brazeau, M. D. The pharynx of the stem-chondrichthyan Ptomacanthus and the early evolution of the gnathostome gill skeleton. Nat. Commun. 10, 2050 (2019).
Hanke, G. F. Paucicanthus vanelsti gen. et sp. nov., an Early Devonian (Lochkovian) acanthodian that lacks paired fin-spines. Can. J. Earth Sci. 39, 1071–1083 (2002).
Brazeau, M. D. The braincase and jaws of a Devonian ‘acanthodian’ and modern gnathostome origins. Nature 457, 305–308 (2009).
Coates, M. I., Gess, R. W., Finarelli, J. A., Criswell, K. E. & Tietjen, K. A symmoriiform chondrichthyan braincase and the origin of chimaeroid fishes. Nature 541, 208–211 (2017).
Brazeau, M. D. et al. Endochondral bone in an Early Devonian 'placoderm' from Mongolia. Nat. Ecol. Evol. 4, 1477–1484 (2020).
Vaškaninová, V. et al. Marginal dentition and multiple dermal jawbones as the ancestral condition of jawed vertebrates. Science 369, 211–216 (2020).
Zhu, Y. A. et al. Endocast and bony labyrinth of a Devonian “placoderm” challenges stem gnathostome phylogeny. Curr. Biol. 31, 1112–1118 (2021).
Smith, M. M. & Sansom, I. J. Exoskeletal micro-remains of an Ordovician fish from the Harding Sandstone of Colorado. Palaeontology 40, 645–658 (1997).
Brazeau, M. D. & Friedman, M. The origin and early phylogenetic history of jawed vertebrates. Nature 520, 490–497 (2015).
Long, J. A., Choo, B. & Clement, A. in Evolution and Development of Fishes (eds Johanson, Z.) 3–29 (Cambridge Univ. Press, 2019).
Sallan, L., Friedman, M., Sansom, R. S., Bird, C. M. & Sansom, I. J. The nearshore cradle of early vertebrate diversification. Science 362, 460–464 (2018).
Wang, N., Donoghue, P. C. J., Smith, M. M. & Sansom, I. J. Histology of the galeaspid dermoskeleton and endoskeleton, and the origin and early evolution of the vertebrate cranial endoskeleton. J. Vert. Paleontol. 25, 745–756 (2005).
Pan, J. Note on Silurian vertebrates of China. Bull. Chin. Acad. Sci. Geol. Sci. 15, 161–190 (1986).
Žigaitė, Ž. & Goujet, D. New observations on the squamation patterns of articulated specimens of Loganellia scotica (Traquair, 1898) (Vertebrata: Thelodonti) from the Lower Silurian of Scotland. Geodiversitas 34, 253–270 (2012).
Hammer, Ø., Bengtson, S., Malzbender, T. & Gelb, D. Imaging fossils using reflectance transformation and interactive manipulation of virtual light sources. Palaeontol. Electron. 5, 1–9 (2002).
King, B., Qiao, T., Lee, M. S. Y., Zhu, M. & Long, J. A. Bayesian morphological clock methods resurrect placoderm monophyly and reveal rapid early evolution in jawed vertebrates. Syst. Biol. 66, 599–516 (2017).
Lloyd, G. T. Estimating morphological diversity and tempo with discrete character–taxon matrices: implementation, challenges, progress, and future directions. Biol. J. Linn. Soc. 118, 131–151 (2016).
Dixon, P. VEGAN, a package of R functions for community ecology. J. Veg. Sci. 14, 927–930 (2003).
Acknowledgements
We thank L. Jia, Q. Wang, Q. Wen, Q. Rao, Y. Zhao, R. Zhao, Q. Xue, Z. Xian, Y. Luo, Y. Yan, H. Wang, Q. Deng, J. Xiong, C. H. Xiong, C. Y. Xiong, J. Zhang, Z. Zhou and L. Nie for fieldwork assistance; J. Rong and Y. Wang for discussion on stratrigraphy; J. Xiong, C. H. Xiong and C. Y. Xiong for fossil preparation; X. Liu and L. Jia for assistance with photography and RTI imaging; Y. Hou and P. Yin for computed microtomography scanning; R. Guo for assistance with drawing and producing the figures; and Q. Zheng for the artistic life reconstruction. This work was supported by the Strategic Priority Research Program of Chinese Academy of Sciences (XDA19050102 and XDB26000000), the National Natural Science Foundation of China (42130209, 42022011, 42072026), the One Hundred Talents Projects of CAS (E0CQ010103) and the Mineral Resources Protection and Supervision Projects of Chongqing (YGZ2020-416). P.E.A. acknowledges the support of a Wallenberg Scholarship from the Knut and Alice Wallenberg Foundation.
Author information
Authors and Affiliations
Contributions
M.Z. designed the project. M.Z., Q.L., G.W., Y.C., J.W., W.Z., Z.G. and Y.Z. conducted the fieldwork, fossil preparation and curation. Y.Z. and J.L. conducted the computed microtomography scans, computed microtomography data segmentation, scanning electron microscopy and RTI; M.Z., J.L. and Y.Z. compiled the character matrix and performed the phylogenetic analyses; Y.Y. helped with the Bayesian analysis using the supercomputing platform and performed the morphospace analyses; Y.Z., M.Z. and P.E.A. contributed to fossil interpretation and wrote the manuscript; Y.Z., J.L., Y.C., J.W. and M.Z. produced the figures. All authors contributed to the interpretation of the results, discussion and manuscript writing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks Matt Friedman and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Locality of Chongqing Lagerstätte.
a, Map showing the location of Chongqing Municipality. b, Map of Chongqing Municipality showing the general location of the fossil locality. c, Geological map of the studied region, showing the locations of the three sections. d, Life restoration of fishes and associated biota in the Chongqing Lagerstätte. Art credit: Qiuyang Zheng.
Extended Data Fig. 2 Silurian stratigraphic columns of the three sections in the studied area yielding fish fossils.
a, Yongdong; b, Tianlu; and c, Kapeng.
Extended Data Fig. 3 Additional fossils from Chongqing Lagerstätte.
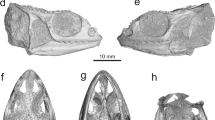
a, Slab bearing the holotypes of Xiushanosteus mirabilis and Shenacanthus vermiformis. b, Interpretative drawing of a. c, Slab bearing a concentration of galeaspids, Xiushanosteus and undescribed jawed vertebrate fossils. d, Interpretative drawing of b. e, Possible vertebrae preserved alongside a placoderm. f, RTI photograph of a complete phyllocarid crustacean. Numbers on the interpretative drawings: 1, galeaspid fossil; 2, Xiushanosteus fossil; unlabeled, undescribed or unidentifiable jawed vertebrate fossils. Scale bars, a-d, 10 mm; e-f, 5 mm.
Extended Data Fig. 4 Additional specimens of X. mirabilis showing the head and trunk shield.
a, Ammonium chloride coated photograph of the negative half of the holotype, V300001b. b, Ammonium chloride coated photograph of a partly flattened specimen showing more sutures, V300010b. c, d, Interpretative drawing of a, b. e, Ammonium chloride coated photograph of V300008b, showing the suture between the preorbital and central plates. f, a specimen showing the cheek and operculum plates, V300002b. g, Interpretative drawing of f. For the abbreviations, see Fig. 2. Scale bars, 5 mm.
Extended Data Fig. 5 Additional specimens of X. mirabilis showing the postthoracic anatomy.
a, Ammonium chloride coated photograph of V300009a. b, Ammonium chloride coated photograph of V300002a, showing the paired fins, the midline scutes and the squamation. c, Ammonium chloride coated photograph of a complete specimen, V300004, showing the two dorsal and caudal fins. d-f, Interpretative drawings of a-c. g, Ammonium chloride coated photograph of a partly articulated specimen missing the anterior part of the head, V300003. h, Interpretative drawing of g. i, V300011, a specimen showing the posterior dorsal and the caudal fins. For the abbreviations, see Fig. 2. Scale bars, 5 mm.
Extended Data Fig. 6 X. mirabilis specimens based on MicroCT scanning.
a-c, Rendering of the holotype V300001a (a), V300007a (b) and V300008a (c) specimens. d-f, Tomographs through the coronal section of the anterior part of above specimens. Not to scale.
Extended Data Fig. 7 Comparison of skull roof patterns in placoderms and osteichthyans.
a, Xiushanosteus. b, Acanthothoracid Romundina. c, Arthrodire Dicksonosteus. d, Maxillate placoderm Bianchengichthys. e, Sarcopterygian osteichthyan Eusthenopteron. f, Actinopterygian osteichthyan Moythomasia. Not to scale.
Extended Data Fig. 8 Additional photographs and energy dispersive spectroscopy of the S. vermiformis holotype (IVPP V300000).
a, The positive half of the holotype, V300000a. b, the head and pectoral region of a. c, Mapping of the concentration of manganese. d, the negative half of the holotype, V300000b. e, Ammonium chloride coated photograph of V300000a. i-j, enlarged ammonium chloride coated photograph of V300000a, showing details of ornament. Scale bar, 5 mm.
Extended Data Fig. 9 Full cladograms of the phylogenetic analyses showing the phylogenetic positions of Xiushanosteus and Shenacanthus.
a, strict consensus tree of the parsimony result, Numbers above branches denote Bremer decay indices larger than zero. b, majority-rule consensus tree of the Bayesian inference analysis.
Extended Data Fig. 10 Additional Plots of the morphospace analysis.
a, morphospace based on the two major axes of variation (NMDS1 and NMDS2), with the colours denotes the five gnathostome groups. b, stress plot denoting the fit of the reduced dimensional space (NMDS1 and NMDS2) to the actual multivariate distance.
Supplementary information
Supplementary Information
The Supplementary Information contains seven sections. (1) Geological context of the Chongqing Lagerstätte. (2) Additional descriptions of Xiushanosteus mirabilis. (3) Additional descriptions of Shenacanthus vermiformis. (4) List of taxa, geological time and references. (5) List of characters. (6) Description of Supplementary Data 1–5 and Supplementary Video 1. (7) Supplementary References.
Supplementary Data 1
Volume renderings of three Xiushanosteus specimens (the holotype IVPP V300001a, IVPP V300007a and IVPP V300008a) based on computed microtomography data. Accessible at Figshare (https://doi.org/10.6084/m9.figshare.20317233.v1).
Supplementary Data 2
Nexus file of the data matrix used in the parsimony and Bayesian phylogenetic analyses.
Supplementary Data 3
The strict consensus tree of the 46 most parsimonious trees resulting from the data matrix of Supplementary Data 1 excluding Xiushanosteus and Shenacanthus.
Supplementary Data 4
Nexus file of the data matrix used in the morphospace analysis. This data matrix is essentially the same as the one used for the phylogenetic analyses, except that the agnathan taxa were expanded.
Supplementary Data 5
Original photographs of the slabs and individual specimens. Accessible at Figshare (https://doi.org/10.6084/m9.figshare.20317233.v1).
Supplementary Video 1
The dawn of fishes. A video reconstructing the fauna, environment and possible taphonomy of the early Silurian Chongqing Lagerstätte.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhu, Ya., Li, Q., Lu, J. et al. The oldest complete jawed vertebrates from the early Silurian of China. Nature 609, 954–958 (2022). https://doi.org/10.1038/s41586-022-05136-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-05136-8
This article is cited by
-
Broad snouted cladoselachian with sensory specialization at the base of modern chondrichthyans
Swiss Journal of Palaeontology (2023)
-
Bony-fish-like scales in a Silurian maxillate placoderm
Nature Communications (2023)
-
Fossil evidence for a pharyngeal origin of the vertebrate pectoral girdle
Nature (2023)
-
How China is capturing attention with landmark research
Nature (2023)
-
How did Jawed Vertebrates Originate and Rise?
Journal of Earth Science (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.