Abstract
Immature dentate granule cells (imGCs) arising from adult hippocampal neurogenesis contribute to plasticity and unique brain functions in rodents1,2 and are dysregulated in multiple human neurological disorders3,4,5. Little is known about the molecular characteristics of adult human hippocampal imGCs, and even their existence is under debate1,6,7,8. Here we performed single-nucleus RNA sequencing aided by a validated machine learning-based analytic approach to identify imGCs and quantify their abundance in the human hippocampus at different stages across the lifespan. We identified common molecular hallmarks of human imGCs across the lifespan and observed age-dependent transcriptional dynamics in human imGCs that suggest changes in cellular functionality, niche interactions and disease relevance, that differ from those in mice9. We also found a decreased number of imGCs with altered gene expression in Alzheimer's disease. Finally, we demonstrated the capacity for neurogenesis in the adult human hippocampus with the presence of rare dentate granule cell fate-specific proliferating neural progenitors and with cultured surgical specimens. Together, our findings suggest the presence of a substantial number of imGCs in the adult human hippocampus via low-frequency de novo generation and protracted maturation, and our study reveals their molecular properties across the lifespan and in Alzheimer's disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The snRNA-seq data are available at the Gene Expression Omnibus database under accession numbers GSE185553, GSE185277 and GSE198323. Specimen information and sequencing statistics are described in Supplementary Tables 1 and 2. Sources of the published scRNA-seq or snRNA-seq datasets used in this study are described in Supplementary Table 3. Source data are provided with this paper.
Code availability
The computational code used in this study is available at GitHub (https://github.com/zhoujoeyyi/humanImmatureNeurons) or upon request.
References
Gage, F. H. Adult neurogenesis in mammals. Science 364, 827–828 (2019).
Ming, G. L. & Song, H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70, 687–702 (2011).
Terreros-Roncal, J. et al. Impact of neurodegenerative diseases on human adult hippocampal neurogenesis. Science 374, 1106–1113 (2021).
Christian, K. M., Song, H. & Ming, G. L. Functions and dysfunctions of adult hippocampal neurogenesis. Annu. Rev. Neurosci. 37, 243–262 (2014).
Moreno-Jimenez, E. P. et al. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer's disease. Nat. Med. 25, 554–560 (2019).
Sorrells, S. F. et al. Positive controls in adults and children support that very few, if any, new neurons are born in the adult human hippocampus. J. Neurosci. 41, 2554–2565 (2021).
Moreno-Jimenez, E. P., Terreros-Roncal, J., Flor-Garcia, M., Rabano, A. & Llorens-Martin, M. Evidences for adult hippocampal neurogenesis in humans. J. Neurosci. 41, 2541–2553 (2021).
Kempermann, G. et al. Human adult neurogenesis: evidence and remaining questions. Cell Stem Cell 23, 25–30 (2018).
Hochgerner, H., Zeisel, A., Lonnerberg, P. & Linnarsson, S. Conserved properties of dentate gyrus neurogenesis across postnatal development revealed by single-cell RNA sequencing. Nat. Neurosci. 21, 290–299 (2018).
Eriksson, P. S. et al. Neurogenesis in the adult human hippocampus. Nat. Med. 4, 1313–1317 (1998).
Spalding, K. L. et al. Dynamics of hippocampal neurogenesis in adult humans. Cell 153, 1219–1227 (2013).
Ernst, A. et al. Neurogenesis in the striatum of the adult human brain. Cell 156, 1072–1083 (2014).
Schmidt-Hieber, C., Jonas, P. & Bischofberger, J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature 429, 184–187 (2004).
Ge, S., Yang, C. H., Hsu, K. S., Ming, G. L. & Song, H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron 54, 559–566 (2007).
Marin-Burgin, A., Mongiat, L. A., Pardi, M. B. & Schinder, A. F. Unique processing during a period of high excitation/inhibition balance in adult-born neurons. Science 335, 1238–1242 (2012).
Knoth, R. et al. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS ONE 5, e8809 (2010).
Boldrini, M. et al. Human hippocampal neurogenesis persists throughout aging. Cell Stem Cell 22, 589–599.e585 (2018).
Tobin, M. K. et al. Human hippocampal neurogenesis persists in aged adults and Alzheimer's disease patients. Cell Stem Cell 24, 974–982.e973 (2019).
Ammothumkandy, A. et al. Altered adult neurogenesis and gliogenesis in patients with mesial temporal lobe epilepsy. Nat. Neurosci. 25, 493–503 (2022).
Sorrells, S. F. et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 555, 377–381 (2018).
Cipriani, S. et al. Hippocampal radial glial subtypes and their neurogenic potential in human fetuses and healthy and Alzheimer's disease adults. Cereb. Cortex 28, 2458–2478 (2018).
Flor-Garcia, M. et al. Unraveling human adult hippocampal neurogenesis. Nat. Protoc. 15, 668–693 (2020).
Kempermann, G., Jessberger, S., Steiner, B. & Kronenberg, G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 27, 447–452 (2004).
Habib, N. et al. Massively parallel single-nucleus RNA-seq with DroNc-seq. Nat. Methods 14, 955–958 (2017).
Ayhan, F. et al. Resolving cellular and molecular diversity along the hippocampal anterior-to-posterior axis in humans. Neuron 109, 2091–2105.e6 (2021).
Davila-Velderrain, J. et al. Single-cell anatomical analysis of human hippocampus and entorhinal cortex uncovers early-stage molecular pathology in Alzheimer’s disease. Preprint at bioRxiv https://doi.org/10.1101/2021.07.01.450715 (2021).
Franjic, D. et al. Transcriptomic taxonomy and neurogenic trajectories of adult human, macaque, and pig hippocampal and entorhinal cells. Neuron 110, 452–469.e414 (2022).
La Manno, G. et al. Molecular diversity of midbrain development in mouse, human, and stem cells. Cell 167, 566–580.e519 (2016).
van Galen, P. et al. Single-cell RNA-seq reveals AML hierarchies relevant to disease progression and immunity. Cell 176, 1265–1281.e1224 (2019).
Telley, L. et al. Temporal patterning of apical progenitors and their daughter neurons in the developing neocortex. Science 364, eaav2522 (2019).
Bielecki, P. et al. Skin-resident innate lymphoid cells converge on a pathogenic effector state. Nature 592, 128–132 (2021).
Shekhar, K. et al. Comprehensive classification of retinal bipolar neurons by single-cell transcriptomics. Cell 166, 1308–1323.e1330 (2016).
Gavet, O. et al. The stathmin phosphoprotein family: intracellular localization and effects on the microtubule network. J. Cell Sci. 111, 3333–3346 (1998).
Zhong, S. et al. Decoding the development of the human hippocampus. Nature 577, 531–536 (2020).
Zhong, S. et al. A single-cell RNA-seq survey of the developmental landscape of the human prefrontal cortex. Nature 555, 524–528 (2018).
Butler, A., Hoffman, P., Smibert, P., Papalexi, E. & Satija, R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat. Biotechnol. 36, 411–420 (2018).
Durante, M. A. et al. Single-cell analysis of olfactory neurogenesis and differentiation in adult humans. Nat. Neurosci. 23, 323–326 (2020).
Lake, B. B. et al. Integrative single-cell analysis of transcriptional and epigenetic states in the human adult brain. Nat. Biotechnol. 36, 70–80 (2018).
Hodge, R. D. et al. Conserved cell types with divergent features in human versus mouse cortex. Nature 573, 61–68 (2019).
Lopez, R., Regier, J., Cole, M. B., Jordan, M. I. & Yosef, N. Deep generative modeling for single-cell transcriptomics. Nat. Methods 15, 1053–1058 (2018).
Qiu, X. et al. Reversed graph embedding resolves complex single-cell trajectories. Nat. Methods 14, 979–982 (2017).
Efremova, M., Vento-Tormo, M., Teichmann, S. A. & Vento-Tormo, R. CellPhoneDB: inferring cell-cell communication from combined expression of multi-subunit ligand-receptor complexes. Nat. Protoc. 15, 1484–1506 (2020).
La Rosa, C., Parolisi, R. & Bonfanti, L. Brain structural plasticity: from adult neurogenesis to immature neurons. Front. Neurosci. 14, 75 (2020).
Jacob, F. et al. A patient-derived glioblastoma organoid model and biobank recapitulates inter- and intra-tumoral heterogeneity. Cell 180, 188–204.e122 (2020).
Kumar, A. et al. Transcriptomic analysis of the signature of neurogenesis in human hippocampus suggests restricted progenitor cell progression post-childhood. IBRO Rep. 9, 224–232 (2020).
Cole, J. D. et al. Adult-born hippocampal neurons undergo extended development and are morphologically distinct from neonatally-born neurons. J. Neurosci. 40, 5740–5756 (2020).
Trinchero, M. F., Herrero, M., Monzon-Salinas, M. C. & Schinder, A. F. Experience-dependent structural plasticity of adult-born neurons in the aging hippocampus. Front. Neurosci. 13, 739 (2019).
Trinchero, M. F. et al. High plasticity of new granule cells in the aging hippocampus. Cell Rep. 21, 1129–1139 (2017).
Kohler, S. J., Williams, N. I., Stanton, G. B., Cameron, J. L. & Greenough, W. T. Maturation time of new granule cells in the dentate gyrus of adult macaque monkeys exceeds six months. Proc. Natl Acad. Sci. USA 108, 10326–10331 (2011).
Ngwenya, L. B., Heyworth, N. C., Shwe, Y., Moore, T. L. & Rosene, D. L. Age-related changes in dentate gyrus cell numbers, neurogenesis, and associations with cognitive impairments in the rhesus monkey. Front. Syst. Neurosci, 9, 102 (2015).
Ferreira, P. G. et al. The effects of death and post-mortem cold ischemia on human tissue transcriptomes. Nat. Commun. 9, 490 (2018).
Terstege, D. J., Addo-Osafo, K., Teskey, G. C. & Epp, J. R. New neurons in old brains: a cautionary tale for the analysis of neurogenesis in post-mortem tissue. Preprint at bioRxiv https://doi.org/10.1101/2021.11.12.468443 (2021).
Rosenberg, A. B. et al. Single-cell profiling of the developing mouse brain and spinal cord with split-pool barcoding. Science 360, 176–182 (2018).
Qian, X. et al. Sliced human cortical organoids for modeling distinct cortical layer formation. Cell Stem Cell 26, 766–781.e769 (2020).
Su, Y. et al. Neuronal activity modifies the chromatin accessibility landscape in the adult brain. Nat. Neurosci. 20, 476–483 (2017).
Macosko, E. Z. et al. Highly parallel genome-wide expression profiling of individual cells using nanoliter droplets. Cell 161, 1202–1214 (2015).
Hu, P. et al. Dissecting cell-type composition and activity-dependent transcriptional state in mammalian brains by massively parallel single-nucleus RNA-seq. Mol. Cell. 68, 1006–1015 e1007 (2017).
Dobin, A. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 (2013).
Hafemeister, C. & Satija, R. Normalization and variance stabilization of single-cell RNA-seq data using regularized negative binomial regression. Genome Biol. 20, 296 (2019).
Pedregosa, F. et al. Scikit-learn: machine learning in Python. J. Mach. Learn. Res. 12, 2825–2830 (2011).
Marques, S. et al. Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 352, 1326–1329 (2016).
Durinck, S., Spellman, P. T., Birney, E. & Huber, W. Mapping identifiers for the integration of genomic datasets with the R/Bioconductor package biomaRt. Nat. Protoc. 4, 1184–1191 (2009).
Lin, Y. et al. Evaluating stably expressed genes in single cells. Gigascience 8, giz106 (2019).
Bindea, G. et al. ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 25, 1091–1093 (2009).
Shannon, P. et al. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Res. 13, 2498–2504 (2003).
Yu, G., Wang, L. G., Han, Y. & He, Q. Y. clusterProfiler: an R package for comparing biological themes among gene clusters. OMICS 16, 284–287 (2012).
Yu, W., Clyne, M., Khoury, M. J. & Gwinn, M. Phenopedia and genopedia: disease-centered and gene-centered views of the evolving knowledge of human genetic associations. Bioinformatics 26, 145–146 (2010).
Doncheva, N. T., Morris, J. H., Gorodkin, J. & Jensen, L. J. Cytoscape StringApp: network analysis and visualization of proteomics data. J. Proteome Res. 18, 623–632 (2019).
Moran, P. A. Notes on continuous stochastic phenomena. Biometrika 37, 17–23 (1950).
Khlghatyan, J. & Saghatelyan, A. Time-lapse imaging of neuroblast migration in acute slices of the adult mouse forebrain. J. Vis. Exp. https://doi.org/10.3791/4061 (2012).
Bardy, C. et al. Neuronal medium that supports basic synaptic functions and activity of human neurons in vitro. Proc. Natl Acad. Sci. USA 112, E2725–E2734 (2015).
Berg, D. A. et al. A common embryonic origin of stem cells drives developmental and adult neurogenesis. Cell 177, 654–668.e615 (2019).
Lange, P. S. et al. ATF4 is an oxidative stress-inducible, prodeath transcription factor in neurons in vitro and in vivo. J. Exp. Med. 205, 1227–1242 (2008).
Palmer, T. D. et al. Progenitor cells from human brain after death. Nature 411, 42–43 (2001).
Zhou, Y. et al. Autocrine Mfge8 signaling prevents developmental exhaustion of the adult neural stem cell pool. Cell Stem Cell 23, 444–452.e444 (2018).
Sun, G. J. et al. Tangential migration of neuronal precursors of glutamatergic neurons in the adult mammalian brain. Proc. Natl Acad. Sci. USA 112, 9484–9489 (2015).
Sun, G. J. et al. Latent tri-lineage potential of adult hippocampal neural stem cells revealed by Nf1 inactivation. Nat. Neurosci. 18, 1722–1724 (2015).
Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682 (2012).
Duque, A., Arellano, J. I. & Rakic, P. An assessment of the existence of adult neurogenesis in humans and value of its rodent models for neuropsychiatric diseases. Mol. Psychiatry 27, 377–382 (2022).
Snyder, J. S. Recalibrating the relevance of adult neurogenesis. Trends Neurosci. 42, 164–178 (2019).
Marchetto, M. C. et al. Species-specific maturation profiles of human, chimpanzee and bonobo neural cells. eLife 8, e37527 (2019).
Acknowledgements
We thank all brain donors, patients and their families for the tissue specimens used in this study and members of Ming and Song laboratories for discussion; B. Temsamrit, E. LaNoce and A. Garcia for technical support; A. Angelucci and J. Schnoll for lab coordination; F. H. Gage, G. Kempermann, S. Jessberger and K. M. Christian for comments; O. Spicer, J. Glausier, A. LeFevre, J. Cottrell and J. Hussain for assistance with human tissue acquisition; K. McCanon, Z. Yang, H. Zhu and CDI Laboratories for providing antibody samples; and M. Llorens-Martin for suggestions on immunohistology. Some of the schematic illustrations were modified and/or created with images from https://togotv.dbcls.jp/ or BioRender.com. This work was supported by grants from the National Institutes of Health (K01MH125144 to A.M.B., R35NS097370 to G.-l.M. and R35NS116843 to H.S.), The Lieber Institute for Brain Development (to J.E.K., T.M.H. and D.R.W.), and Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (to G.-l.M.).
Author information
Authors and Affiliations
Contributions
Y.Z. performed bioinformatics and immunohistological analyses and slice culture experiments. Y. Su performed snRNA-seq experiments. S.L. performed snRNA-seq library generation with the help of L.L. D.Y.Z. performed cell-interaction analysis. A.M.B. contributed to the ex vivo slice culture idea. F.J. contributed to the initial slice culture setup. B.C.K., T.L. and H.I.C. provided surgical specimens for slice culture with the coordination of I.H., S.K.K. and R.D.S. A.N.V. and D.W.N. provided some of the human postmortem specimens for immunohistological validation. B.C.K., J.E.K., T.M.H. and D.R.W. provided some of the human specimens for snRNA-seq. Y. Sun, P.H., H.W. and X.G. contributed to additional data collection. Y.Z., Y. Su, G-l.M. and H.S. conceived the project and wrote the manuscript with inputs from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Characteristics of the snRNA-seq dataset of the infant human hippocampus.
a, Expression patterns of marker genes used to determine cluster identities. Ex.: excitatory; OPC: oligodendrocyte precursor cells. b, Uniform Manifold Approximation and Projection (UMAP) visualization of all cells from the four infant hippocampi (0-2 years) colored by specimen. HIP: hippocampus; yrs: years. c, UMAP plots of nuclei from four human infant hippocampal specimens by marker gene expression. The dentate granule cell cluster is highlighted with a dashed line circle.
Extended Data Fig. 2 Machine learning model trained with the mouse early postnatal hippocampal scRNA-seq dataset.
a, b, Unsupervised clustering and t-distributed Stochastic Neighbor Embedding (t-SNE) visualization of all cells from the mouse postnatal (P5) hippocampus9 colored by cluster (a) and marker gene expression (b). imGC: immature dentate granule cell; GC: dentate granule cell; IPC: intermediate progenitor cell; OPC: oligodendrocyte precursor cell. RGL: radial glia-like cell; VLMC: vascular and leptomeningeal cell. c, A schematic illustration of the machine learning-aided analysis using the mouse hippocampal scRNA-seq datasets9, mirroring our analysis pipeline in human studies (Fig. 1a). In brief, Dcx+Calb1−Prox1+ imGCs in the P5 mouse dentate gyrus were selected as prototypes to train a scoring model to comprehensively learn their gene features. The trained model containing an aggregate of weighted features (“gene weights”) was then used to quantitatively evaluate the similarity of each cell to the imGC prototype in query (test) datasets of the early postnatal (P5; self-scoring), the juvenile (P12-35) and the adult (P120-132) hippocampus9. To assess the efficacy of our method, we classified cells with high similarity scores to the imGC prototype as imGCs and compared our model classifications to the published annotations based on unsupervised clustering9 (Shown in Extended Data Fig. 3). d, Measuring performance of the machine learning model. Line plot showing the accuracy score of the machine learning classifier varying with decreasing regularization strength as estimated by cross-validation. Red line shows 95% confidence interval on the estimation of the accuracy score. #Sum abs (coeffs): sum of the absolute value of regression coefficients. e, Heatmap showing expression of top-weighted genes in top-scoring cells of each prototype determined by the machine learning model. Genes listed are the top 25 weights defining mouse imGCs. f, Wheel plot visualizing the scores of each cell to each prototype. Dots represent individual cells whose distance to each prototype is proportional to the score of that prototype. Red and lime green dots represent the prototypical imGCs and all other GCs, respectively. Dotted line indicates a similarity score of 0.85 to each prototypical cell type. Note that unlike in the human system (Fig. 1c), no mature oligodendrocyte (mOli) cluster was present in the P5 mouse hippocampus.
Extended Data Fig. 3 Validation of prototype-based scoring of mouse imGCs across ages by the trained machine learning model with published annotations based on unsupervised clustering.
a, b, d, e, g, h, t-SNE visualization of previously published mouse hippocampal datasets9 at postnatal (a), juvenile (d), and adult (g) stages, colored by four broad cell classes and by similarity score to prototypical imGCs (b, e, h). c, f, i, Benchmarking cells with high similarity scores (P ≥ 0.85) with the published annotations9. Percentage of cells in the GC lineage clusters (based on published annotations9) that are selected as imGCs by our trained machine learning model are indicated in red, bold text.
Extended Data Fig. 4 Machine learning model performance and feature extraction of gene weights defining human imGCs.
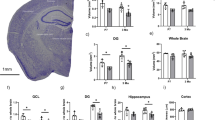
a, Efficacy of the machine-learning approach. Line plot showing the accuracy score of the machine learning model varying with decreasing regularization strength as estimated by cross-validation. Red line shows 95% confidence interval on the estimation of the accuracy score. b, Heatmap showing expression of top gene weights in top-scoring cells of each prototype determined by the machine learning model. Genes listed are the top 15 weights defining human imGCs. c, Gene ontology (GO) network of biological processes of the positive gene weights defining human imGCs, colored by functionally related ontology group. Only significantly enriched nodes are displayed (one-sided hypergeometric test, false-discovery rate-adjust p value (FDR) < 0.05). The node size represents the term enrichment significance. Examples of the most significant terms per group are shown. See also Supplementary Table 5 for the list of GO terms. d, Functional protein-protein association network68 of the positive gene weights defining human imGCs, highlighting the first-degree neighbors (high-confidence connections) in orange related to DCX. e, Overlap of the positive gene weights defining imGCs in humans and in mice that were generated by separate machine learning models. See Supplementary Table 4 for the lists of genes. f, g, Immunohistological analysis showing Stmn1 enrichment in immature neurons in the adult mouse dentate gyrus. Shown are sample confocal images (f) and quantification (g) of Stmn1 expression in imGCs in the adult mouse hippocampus. Individual dots represent value of quantification for different sections (f). Scale bars, 10 µm. Box plots similar as in Fig. 1g (n = 4 mice) (g).
Extended Data Fig. 5 Specificity of the machine learning approach for identification of human immature neurons.
The fractions of cells with high similarity scores (P ≥ 0.85) among non-GC excitatory neuron (a), GABA interneuron (b), and non-neuronal cell (c) clusters in various scRNA-seq or snRNA-seq datasets of the human brains. Box plots represent mean ± s.e.m. with whiskers for max and min. See Supplementary Tables 1, 2, 3 for the specimens used in ours and all published datasets.
Extended Data Fig. 6 Immunohistological analysis of STMN1 enrichment in human imGCs across the lifespan.
a-d, Sample confocal images (a, b) and quantifications (c, d) of imGCs in the human dentate gyrus across the lifespan. Asterisks indicate DCX+ or CALB1- among STMN1+PROX1+ GCs (a, b). Box plots similar as in Fig. 1g (n = 4 subjects each group) (c, d). The immunohistological signal of STMN1 was noticeably more robust than that of DCX in adult specimens. e, f, Sample confocal images showing NEUROD1+, NEUN+ (e), S100B−, or OLIG2− (f) among STMN1+PROX1+ imGCs in infant or adult human dentate gyrus, confirming their neuronal identity. Asterisks indicate STMN1+PROX1+ imGCs (n = 1 specimen for each immunostaining). All scale bars, 10 µm.
Extended Data Fig. 7 Age-dependent transcriptomic dynamics are specific to human imGCs.
a-c, In contrast to human imGCs (Fig. 3f), pseudo-age cell alignment of human mature (a), mouse immature (b), and mouse mature (c) GCs9 shows very little age-related divergence, visualized as scatter plots. Cells were colored by age group. Distribution of cells within each age group on the pseudo-age trajectory is displayed in the density plots (bottom left). See summary plots in Fig. 3g. d, Summary plot comparing pseudo-age alignment (y-axis) of mouse mGCs to real age groups (x-axis), with each mGC of the different age groups plotted as a data point in the background. Data points are fitted with loess fitting (lines) with 95% confidence interval (grey shades). Pearson’s r was measured for correlation of pseudo-age and real-age groups. Mouse datasets9 at prenatal (E16.5), neonatal (P0), or early postnatal (P5) stages do not contain mGC populations.
Extended Data Fig. 8 Consistent expression of Neurod4 and Nfia in imGCs of the postnatal mouse hippocampus across ages.
a, b, Sample confocal immunostaining images (a) and quantification (b) of two exemplary genes that display age-dependent expression patterns in human imGCs (Fig. 3j), but consistent expression in mouse imGCs across ages. Scale bar, 10 µm (a). Box plots similar as in Fig. 1g (n = 3 mice per age group) (b).
Extended Data Fig. 9 Characterization of the slice culture system.
a, b, Sample confocal images (a) and quantification (b) of cell death and oxidative stress level measured in our human hippocampal slice culture in comparison to the post-mortem tissue, using immunohistological analysis of cleaved Caspase 3 and ATF4 (a marker of oxidative stress73), respectively. Dots represent value of quantification for individual sections and boxes represent mean ± s.e.m with whiskers for max and min (n = 2 sections) (b). c, Sample confocal immunostaining images showing baseline cellular composition of slice culture and post-mortem tissue. NEUN+ neurons, IBA1+ microglia, S100B+ astrocytes, and OLIG2+ oligodendrocyte lineage cells were observed. d, Sample confocal images showing EdU-incorporated PROX1+ newborn GCs are absent of the astrocyte marker S100B or the more mature neuron marker CALB1 in slice cultures. Asterisks indicate EdU+PROX1+ GCs. For c, d, n = 1 section for each immunostaining. Scale bars: 50 µm for main panels and 10 µm for insets. e, Quantification of EdU-incorporated newborn imGCs expressing different markers in slice culture of the postnatal human dentate gyrus. Box plots similar as in Fig. 1g (n = 4 subjects).
Extended Data Fig. 10 Protracted neuronal maturation leads to accumulation of immature neurons in the presence of low frequency of de novo new neuron generation.
a, Process of adult hippocampal neurogenesis1,2. Proliferating intermediate progenitor cells (IPCs) and neuroblasts (brown) arising from activated neural stem cells (NSCs, grey) generate new post-mitotic immature dentate granule cells (imGCs, red), which develop over time into mature dentate granule cells (mGCs, lime-green). b, An “imGC protracted maturation” model explaining how low-rate, continuous IPC generation can lead to a large number of imGCs as a reservoir, as opposed to a “fast maturation” model. The size of the imGC reservoir in the adult hippocampus depends on a number of factors at the cellular level, such as the rate of stem cell activation and IPC generation, the number of progeny each IPC generates, the percentage of progeny that survives79, and the duration of imGCs remaining in the immature state, and these parameters may vary tremendously across species and ages80. Here we illustrate side-by-side two schematic models showing how changing one factor, the length of imGC maturation duration, alone while keeping all other parameters the same can lead to significant differences in the outcome on the number of imGCs at a given time. For IPCs in a newly generated cohort at a given time t, they go through stereotypical developmental stages to become imGCs and then mGCs (x-axis). At time t+1, a new IPC cohort is generated (y-axis). With all other parameters the same, if the imGCs mature fast, very few imGCs will be observed at any given time (left model). In contrast, if the average length of imGC maturation duration is substantially longer, imGCs in various maturation stages accumulate over time and are present as a large population in any “snapshot” (right model). Prolonged maturation duration of new neurons in the hippocampus has been demonstrated in non-human primates using nucleotide analog tracing analysis to be at least six months49 and over a year50. Furthermore, human induced pluripotent stem cell-derived transplanted neurons display significantly slower maturation compared to those of three non-human primates81. c, d, An indifference curve qualitatively depicting different combinations of two factors, the average rate of new neuron generation (\(\bar{{\rm{r}}}\)g) and the average duration of imGC maturation (\(\bar{{\rm{t}}}\)d), to achieve an equal size of imGC reservoirs (c). Hypothetical examples shown in d. A significantly longer \(\bar{{\rm{t}}}\)d in the adult human hippocampus spares the system from high demand of \(\bar{{\rm{r}}}\)g to maintain the same size of imGC reservoir, which is a potential model to explain the seemingly counterintuitive discrepancy between the few IPCs and a large number of imGCs in our results.
Supplementary information
Supplementary Figures
Supplementary Figs 1–6 and Supplementary References.
Supplementary Table 1
Summary of human specimens used in the current study.
Supplementary Table 2
Summary of sequencing characteristics of the single-nucleus RNA-seq datasets.
Supplementary Table 3
Summary of published single-cell or single-nucleus RNA-seq datasets used in the current study.
Supplementary Table 4
Gene weights defining immature dentate granule cells in humans and in mice.
Supplementary Table 5
Gene Ontology terms related to the positive gene weights defining human immature dentate granule cells.
Supplementary Table 6
List of genes and Gene Ontology terms enriched in human immature dentate granule cells relative to mature granule cells.
Supplementary Table 7
Lists of common and divergent genes enriched in immature dentate granule cells in humans and in mice.
Supplementary Table 8
Lists of Gene Ontology terms for the five patterns of functional programmes of human immature dentate granule cells across ages.
Supplementary Table 9
Lists of differentially expressed genes in imGCs between Alzheimer's disease samples and matched controls and the associated Gene Ontology terms.
Rights and permissions
About this article
Cite this article
Zhou, Y., Su, Y., Li, S. et al. Molecular landscapes of human hippocampal immature neurons across lifespan. Nature 607, 527–533 (2022). https://doi.org/10.1038/s41586-022-04912-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-022-04912-w
This article is cited by
-
The concept of resilience to Alzheimer’s Disease: current definitions and cellular and molecular mechanisms
Molecular Neurodegeneration (2024)
-
Genetics of human brain development
Nature Reviews Genetics (2024)
-
The impact of adult neurogenesis on affective functions: of mice and men
Molecular Psychiatry (2024)
-
Protracted neuronal recruitment in the temporal lobes of young children
Nature (2024)
-
Formation of memory assemblies through the DNA-sensing TLR9 pathway
Nature (2024)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.