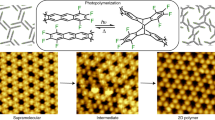
Abstract
Polymers that extend covalently in two dimensions have attracted recent attention1,2 as a means of combining the mechanical strength and in-plane energy conduction of conventional two-dimensional (2D) materials3,4 with the low densities, synthetic processability and organic composition of their one-dimensional counterparts. Efforts so far have proven successful in forms that do not allow full realization of these properties, such as polymerization at flat interfaces5,6 or fixation of monomers in immobilized lattices7,8,9. Another frequently employed synthetic approach is to introduce microscopic reversibility, at the cost of bond stability, to achieve 2D crystals after extensive error correction10,11. Here we demonstrate a homogenous 2D irreversible polycondensation that results in a covalently bonded 2D polymeric material that is chemically stable and highly processable. Further processing yields highly oriented, free-standing films that have a 2D elastic modulus and yield strength of 12.7 ± 3.8 gigapascals and 488 ± 57 megapascals, respectively. This synthetic route provides opportunities for 2D materials in applications ranging from composite structures to barrier coating materials.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Sakamoto, J., van Heijst, J., Lukin, O. & Schlüter, A. D. Two-dimensional polymers: just a dream of synthetic chemists? Angew. Chem. Int. Ed. 48, 1030–1069 (2009).
Schlüter, A. D., Payamyar, P. & Öttinger, H. C. How the world changes by going from one- to two-dimensional polymers in solution. Macromol. Rapid Commun. 37, 1638–1650 (2016).
Lee, C., Wei, X., Kysar, J. W. & Hone, J. Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 321, 385–388 (2008).
Pop, E., Varshney, V. & Roy, A. K. Thermal properties of graphene: fundamentals and applications. MRS Bull. 37, 1273–1281 (2012).
Stupp, S. I., Son, S., Lin, H. C. & Li, L. S. Synthesis of two-dimensional polymers. Science 259, 59–63 (1993).
Grill, L. et al. Nano-architectures by covalent assembly of molecular building blocks. Nat. Nanotechnol. 2, 687–691 (2007).
Kissel, P. et al. A two-dimensional polymer prepared by organic synthesis. Nat. Chem. 4, 287–291 (2012).
Kissel, P., Murray, D. J., Wulftange, W. J., Catalano, V. J. & King, B. T. A nanoporous two-dimensional polymer by single-crystal-to-single-crystal photopolymerization. Nat. Chem. 6, 774–778 (2014).
Kory, M. J. et al. Gram-scale synthesis of two-dimensional polymer crystals and their structure analysis by X-ray diffraction. Nat. Chem. 6, 779–784 (2014).
Kandambeth, S., Dey, K. & Banerjee, R. Covalent organic frameworks: chemistry beyond the structure. J. Am. Chem. Soc. 141, 1807–1822 (2019).
Diercks, C. S. & Yaghi, O. M. The atom, the molecule, and the covalent organic framework. Science 355, eaal1585 (2017).
Baek, K. et al. Free-standing, single-monomer-thick two-dimensional polymers through covalent self-assembly in solution. J. Am. Chem. Soc. 135, 6523–6528 (2013).
Gee, G. & Rideal, E. K. Reaction in monolayers of drying oils I—the oxidation of the maleic anhydride compound of β-elaeostearin. Proc. R. Soc. Lond. A 153, 116–128 (1935).
Ozaki, H. et al. Formation of atomic cloth observed by Penning ionization electron spectroscopy. J. Chem. Phys. 103, 1226–1228 (1995).
Zhong, Y. et al. Wafer-scale synthesis of monolayer two-dimensional porphyrin polymers for hybrid superlattices. Science 366, 1379–1384 (2019).
Côté, A. P. et al. Porous, crystalline, covalent organic frameworks. Science 310, 1166–1170 (2005).
Berlanga, I. et al. Delamination of layered covalent organic frameworks. Small 7, 1207–1211 (2011).
Zhang, B. et al. Crystalline dioxin-linked covalent organic frameworks from irreversible reactions. J. Am. Chem. Soc. 140, 12715–12719 (2018).
Guan, X. et al. Chemically stable polyarylether-based covalent organic frameworks. Nat. Chem. 11, 587–594 (2019).
Cai, Z., Liu, B., Zou, X. & Cheng, H. M. Chemical vapor deposition growth and applications of two-dimensional materials and their heterostructures. Chem. Rev. 118, 6091–6133 (2018).
Zhang, G., Zeng, Y., Gordiichuk, P. & Strano, M. S. Chemical kinetic mechanisms and scaling of two-dimensional polymers via irreversible solution-phase reactions. J. Chem. Phys. https://doi.org/10.1063/5.0044050 (2021).
Payamyar, P., King, B. T., Öttinger, H. C. & Schlüter, A. D. Two-dimensional polymers: concepts and perspectives. Chem. Commun. 52, 18–34 (2016).
Varoon, K. et al. Dispersible exfoliated zeolite nanosheets and their application as a selective membrane. Science 334, 72–75 (2011).
Yeh, T.-M., Wang, Z., Mahajan, D., Hsiao, B. S. & Chu, B. High flux ethanol dehydration using nanofibrous membranes containing graphene oxide barrier layers. J. Mater. Chem. A 1, 12998–13003 (2013).
Li, P. et al. In situ nanomechanical characterization of multi-layer MoS2 membranes: from intraplanar to interplanar fracture. Nanoscale 9, 9119–9128 (2017).
Bunch, J. S. et al. Impermeable atomic membranes from graphene sheets. Nano Lett. 8, 2458–2462 (2008).
Sun, P. Z. et al. Limits on gas impermeability of graphene. Nature 579, 229–232 (2020).
Leterrier, Y. Durability of nanosized oxygen-barrier coatings on polymers. Prog. Mater Sci. 48, 1–55 (2003).
Fang, Q. et al. Strong and flaw-insensitive two-dimensional covalent organic frameworks. Matter 4, 1017–1028 (2021).
Sandoz-Rosado, E., Beaudet, T. D., Andzelm, J. W. & Wetzel, E. D. High strength films from oriented, hydrogen-bonded “graphamid” 2D polymer molecular ensembles. Sci. Rep. 8, 3708 (2018).
Liu, P. et al. Layered and scrolled nanocomposites with aligned semi-infinite graphene inclusions at the platelet limit. Science 353, 364–367 (2016).
Acknowledgements
Membraackackackne fabrication, permeability testing and transport analysis of 2DPA-1 was supported by the Center for Enhanced Nanofluidic Transport (CENT), an Energy Frontier Research Center sponsored by the US Department of Energy (DOE), Office of Science, Basic Energy Sciences under award #DE-SC0019112. The synthetic chemistry and mechanical testing aspects of this work were funded by the Army Research Laboratory under cooperative agreement W911NF-18-2-0055. H.J.K. holds a Career Award at the Scientific Interface from the Burroughs Wellcome Fund, which supported the molecular modelling aspects of this work. We acknowledge fabrication support from the Center for Nanoscale Systems at Harvard, a member of the National Nanotechnology Coordinated Infrastructure Network (NNCI), which is supported by the National Science Foundation under NSF award no. 1541959. This research used beamline 11-BM Complex Materials Scattering (CMS) of the National Synchrotron Light Source II (NSLS-II) and the Center for Functional Nanomaterials (CFN), both of which are US DOE Office of Science User Facilities operated for the DOE Office of Science by Brookhaven National Laboratory under contract no. DE-SC0012704. We thank E. Tsai for her assistance in performing experiments at the beamline, R. Verduzco for beamline access, A. Penn and E. Brignole for MIT.Nano assistance for STEM and Cryo EM, and S. Xin Li for discussions on 2D membrane properties. We acknowledge MIT.Nano facilities and the Cypher VRS DURIP award (N000142012203) for the support on AFM characterizations. M.K. acknowledges support by the German Research Foundation (DFG) Research Fellowship KU 3952/1-1.
Author information
Authors and Affiliations
Contributions
Y.Z. and M.S.S. conceived and designed the reaction system, with initial laboratory synthesis and characterization of 2DPA-1 performed by Y.Z. Characterization using HR-AFM measurement and other imaging tools, and subsequent data analysis, was performed by Y.Z., P.G., X.G. and J.T. P.L., A.T.L., and J.F.Y. offered substrates for AFM characterization. Post-synthesis modification for molecular imaging and intermediate characterization was performed by Y.Z.; M.Q. and D.J.L. contributed to data analysis. Y.Z. and M.S.S. designed and developed the NMR characterization technique for defect quantification. Y.Z., M.Q. and G.Z. performed budge tests. Z. Yuan, G.Z., G.H. and M.Q. calculated permeabilities for 2DPA-1 from bulge test results. Y.Z. performed nanoindentation tests at MIT with complementary measurements from E.D.W. and E.S.-R. at ARL. Substrates for nanoindentation tests are designed and fabricated by M.K. Y.Z. designed and T.I. conducted polarized photoluminescence measurements. Y.Z. measured mechanical properties of scrolled fibres. G.Z., D.K., J. Yang, M.S.S. and H.J.K. contributed theory and material simulation. Z. Yang and H.J.K. performed molecular structure calculations for 2DPA-1. Y.Z. and M.S.S. co-wrote the manuscript with input from all other authors.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review information
Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Schematic illustration of rotation suppression and auto-catalysis.
a, Linkage-core conjugations inhibit out-of-plane rotation. b, Auto-catalytic self-templating. Computational method: gas-phase geometry optimizations with Q-Chem v4.2 to compute 298 K conjugation enthalpies using the ωB97-XD/6-311 + G(d, p) DFT functional and basis set combination.
Extended Data Fig. 2 Characterization of 2DPA-1.
a, Fourier-transform infrared (FTIR) spectrum of as-synthesized 2DPA-1 powder. b, Atomic force microscopy (AFM) image of bilayer nanoclusters and its height histogram along the white line (inset). c, AFM image of stacked nanosheets; inset shows its height histogram along the white line. d, AFM surface topology of a spin-coated film. e, AFM image from amplitude channel at its second eigenmode, obtained from a film surface. f, The size distribution obtained from e.
Extended Data Fig. 3 Silylation of 2DPA-1.
a, Synthetic scheme of the silylation reaction. b, FTIR spectra of reaction mixture and its starting material. rt, room temperature; TMSOTf, trimethylsilyl trifluoromethanesulfonate; TEA, triethylamine.
Extended Data Fig. 4 Bulge test of 2DPA-1 films for air permeability.
a, Cross-sectional view of a clean holey substrate. b, Bubble height versus time. Film thickness is 12.8 nm.
Extended Data Fig. 5 Scrolled fibre tensile test of 2DPA-1 composites.
a, Schematic illustration of an Archimedean scroll fibre. b, Optical micrograph of a hair (left) and a scrolled fibre (right). Scale bar, 100 mm. c, Representative true stress–strain curves from a 2D composite scrolled fibre, its polycarbonate (PC) control fibre, and a graphene/PC composite fibre (data reproduced from ref. 31). The volume fraction for 2DPA-1/PC is 6.9% and for graphene/PC it is 0.19%. d, Plot of modulus enhancement ((E − EPC)/EPC) versus different volume fractions of 2DPA-1.
Extended Data Fig. 6 True stress–strain plots of composite scrolled fibres and their PC controls.
a, Volume fraction (V2DP) = 0.9%. b, V2DP = 2.3%. c, V2DP = 6.9%. d, V2DP = 7.7%. e, V2DP = 13.3%.
Extended Data Fig.7 Optical set-up for photoluminescence measurements.
Excitation wavelength 532 nm, excitation power 500 μW for photoluminescence measurements, and 2 μW for excitation polarization. EMCCD, electron-multiplying charge-coupled device.
Supplementary information
Supplementary Information
This file contains Supplementary Information, including Supplementary Figures 1–45, Supplementary Tables 1–3, and additional references.
Rights and permissions
About this article
Cite this article
Zeng, Y., Gordiichuk, P., Ichihara, T. et al. Irreversible synthesis of an ultrastrong two-dimensional polymeric material. Nature 602, 91–95 (2022). https://doi.org/10.1038/s41586-021-04296-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-04296-3
This article is cited by
-
Research progresses of nanomaterials as lubricant additives
Friction (2024)
-
What is conceptual disruption?
Ethics and Information Technology (2024)
-
A self-standing three-dimensional covalent organic framework film
Nature Communications (2023)
-
Fundamental Understanding and Optimization Strategies for Dual-Ion Batteries: A Review
Nano-Micro Letters (2023)
-
Robust and flexible free-standing polyimide/SiOx nanocomposite one-dimensional photonic crystals with high reflectance
Journal of Materials Science (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.