Abstract
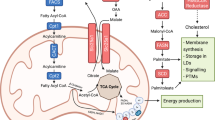
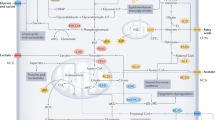
Dietary interventions can change metabolite levels in the tumour microenvironment, which might then affect cancer cell metabolism to alter tumour growth1,2,3,4,5. Although caloric restriction (CR) and a ketogenic diet (KD) are often thought to limit tumour progression by lowering blood glucose and insulin levels6,7,8, we found that only CR inhibits the growth of select tumour allografts in mice, suggesting that other mechanisms contribute to tumour growth inhibition. A change in nutrient availability observed with CR, but not with KD, is lower lipid levels in the plasma and tumours. Upregulation of stearoyl-CoA desaturase (SCD), which synthesises monounsaturated fatty acids, is required for cancer cells to proliferate in a lipid-depleted environment, and CR also impairs tumour SCD activity to cause an imbalance between unsaturated and saturated fatty acids to slow tumour growth. Enforcing cancer cell SCD expression or raising circulating lipid levels through a higher-fat CR diet confers resistance to the effects of CR. By contrast, although KD also impairs tumour SCD activity, KD-driven increases in lipid availability maintain the unsaturated to saturated fatty acid ratios in tumours, and changing the KD fat composition to increase tumour saturated fatty acid levels cooperates with decreased tumour SCD activity to slow tumour growth. These data suggest that diet-induced mismatches between tumour fatty acid desaturation activity and the availability of specific fatty acid species determine whether low glycaemic diets impair tumour growth.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated or analysed in this study are included in the published article and in Supplementary Table 1, Supplementary Figs. 1–23 and Source Data for Figs. 1–4 and Extended Data Figs. 1–8. Correspondence and requests for materials should be addressed to Matthew G. Vander Heiden (mvh@mit.edu). Source data are provided with this paper.
References
Lien, E. C. & Vander Heiden, M. G. A framework for examining how diet impacts tumour metabolism. Nat. Rev. Cancer 19, 651–661 (2019).
Sullivan, M. R. et al. Increased serine synthesis provides an advantage for tumors arising in tissues where serine levels are limiting. Cell Metab. 29, 1410–1421 (2019).
Sullivan, M. R. et al. Quantification of microenvironmental metabolites in murine cancers reveals determinants of tumor nutrient availability. Elife 8, e44235 (2019).
Maddocks, O. D. K. et al. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature 544, 372–376 (2017).
Gao, X. et al. Dietary methionine influences therapy in mouse cancer models and alters human metabolism. Nature 572, 397–401 (2019).
Kalaany, N. Y. & Sabatini, D. M. Tumours with PI3K activation are resistant to dietary restriction. Nature 458, 725–731 (2009).
Hopkins, B. D. et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature 560, 499–503 (2018).
Nencioni, A., Caffa, I., Cortellino, S. & Longo, V. D. Fasting and cancer: molecular mechanisms and clinical application. Nat. Rev. Cancer 18, 707–719 (2018).
Danai, L. V. et al. Altered exocrine function can drive adipose wasting in early pancreatic cancer. Nature 558, 600–604 (2018).
Gocheva, V. et al. Quantitative proteomics identify Tenascin-C as a promoter of lung cancer progression and contributor to a signature prognostic of patient survival. Proc. Natl Acad. Sci. USA 114, E5625–E5634 (2017).
Kennedy, A. R. et al. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am. J. Physiol. Endocrinol. Metab. 292, E1724–E1739 (2007).
Mukherjee, P., El-Abbadi, M. M., Kasperzyk, J. L., Ranes, M. K. & Seyfried, T. N. Dietary restriction reduces angiogenesis and growth in an orthotopic mouse brain tumour model. Br. J. Cancer 86, 1615–1621 (2002).
Zhang, J. et al. Low ketolytic enzyme levels in tumors predict ketogenic diet responses in cancer cell lines in vitro and in vivo. J. Lipid Res. 59, 625–634 (2018).
Hosios, A. M., Li, Z., Lien, E. C. & Vander Heiden, M. G. Preparation of lipid-stripped serum for the study of lipid metabolism in cell culture. Bio Protoc. 8, e2876 (2018).
Peck, B. & Schulze, A. Lipid desaturation—the next step in targeting lipogenesis in cancer. FEBS J. 283, 2767–2778 (2016).
Vriens, K. et al. Evidence for an alternative fatty acid desaturation pathway increasing cancer plasticity. Nature 566, 403–406 (2019).
Kim, W. et al. Polyunsaturated fatty acid desaturation is a mechanism for glycolytic NAD+ recycling. Cell Metab. 29, 856–870 (2019).
Sullivan, L. B. et al. Supporting aspartate biosynthesis is an essential function of respiration in proliferating cells. Cell 162, 552–563 (2015).
Birsoy, K. et al. An essential role of the mitochondrial electron transport chain in cell proliferation is to enable aspartate synthesis. Cell 162, 540–551 (2015).
Titov, D. V. et al. Complementation of mitochondrial electron transport chain by manipulation of the NAD+/NADH ratio. Science 352, 231–235 (2016).
Diehl, F. F., Lewis, C. A., Fiske, B. P. & Vander Heiden, M. G. Cellular redox state constrains serine synthesis and nucleotide production to impact cell proliferation. Nat. Metab. 1, 861–867 (2019).
Piccolis, M. et al. Probing the global cellular responses to lipotoxicity caused by saturated fatty acids. Mol. Cell 74, 32–44 (2019).
Mauvoisin, D. & Mounier, C. Hormonal and nutritional regulation of SCD1 gene expression. Biochimie 93, 78–86 (2011).
Young, R. M. et al. Dysregulated mTORC1 renders cells critically dependent on desaturated lipids for survival under tumor-like stress. Genes Dev. 27, 1115–1131 (2013).
Ackerman, D. et al. Triglycerides promote lipid homeostasis during hypoxic stress by balancing fatty acid saturation. Cell Rep. 24, 2596–2605 (2018).
Kamphorst, J. J. et al. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc. Natl Acad. Sci. USA 110, 8882–8887 (2013).
Coulston, A. M. The role of dietary fats in plant-based diets. Am. J. Clin. Nutr. 70, 512S–515S (1999).
Pascual, G. et al. Targeting metastasis-initiating cells through the fatty acid receptor CD36. Nature 541, 41–45 (2017).
Siri-Tarino, P. W., Chiu, S., Bergeron, N. & Krauss, R. M. Saturated fats versus polyunsaturated fats versus carbohydrates for cardiovascular disease prevention and treatment. Annu. Rev. Nutr. 35, 517–543 (2015).
Mayers, J. R. et al. Tissue of origin dictates branched-chain amino acid metabolism in mutant Kras-driven cancers. Science 353, 1161–1165 (2016).
Lewis, C. A. et al. Tracing compartmentalized NADPH metabolism in the cytosol and mitochondria of mammalian cells. Mol. Cell 55, 253–263 (2014).
Heinrich, P. et al. Correcting for natural isotope abundance and tracer impurity in MS-, MS/MS- and high-resolution-multiple-tracer-data from stable isotope labeling experiments with IsoCorrectoR. Sci. Rep. 8, 17910 (2018).
Antoniewicz, M. R., Kelleher, J. K. & Stephanopoulos, G. Measuring deuterium enrichment of glucose hydrogen atoms by gas chromatography/mass spectrometry. Anal. Chem. 83, 3211–3216 (2011).
Argus, J. P. et al. Development and application of FASA, a model for quantifying fatty acid metabolism using stable isotope labeling. Cell Rep. 25, 2919–2934 (2018).
Vichai, V. & Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 1, 1112–1116 (2006).
Colditz, G. A. & Hankinson, S. E. The Nurses’ Health Study: lifestyle and health among women. Nat. Rev. Cancer 5, 388–396 (2005).
Belanger, C. F., Hennekens, C. H., Rosner, B. & Speizer, F. E. The nurses’ health study. Am. J. Nurs. 78, 1039–1040 (1978).
Health Professionals Follow-Up Study (Harvard T. H. Chan School of Public Health, 2020); https://sites.sph.harvard.edu/hpfs/
Hu, F. B. et al. Reproducibility and validity of dietary patterns assessed with a food-frequency questionnaire. Am. J. Clin. Nutr. 69, 243–249 (1999).
Feskanich, D. et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J. Am. Diet Assoc. 93, 790–796 (1993).
Willett, W. C. et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am. J. Epidemiol. 122, 51–65 (1985).
Rimm, E. B. et al. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am. J. Epidemiol. 135, 1114–1126 (1992).
Willett, W. C. Nutritional Epidemiology (Oxford University Press, 2012).
Yuan, C. et al. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am. J. Epidemiol. 185, 570–584 (2017).
Yuan, C. et al. Relative validity of nutrient intakes assessed by questionnaire, 24-hour recalls, and diet records as compared with urinary recovery and plasma concentration biomarkers: findings for women. Am. J. Epidemiol. 187, 1051–1063 (2018).
Halton, T. L., Liu, S., Manson, J. E. & Hu, F. B. Low-carbohydrate-diet score and risk of type 2 diabetes in women. Am. J. Clin. Nutr. 87, 339–346 (2008).
Halton, T. L. et al. Low-carbohydrate-diet score and the risk of coronary heart disease in women. N. Engl. J. Med. 355, 1991–2002 (2006).
Liu, Y. et al. Plant-based and animal-based low-carbohydrate diets and risk of hepatocellular carcinoma among US men and women. Hepatology 73, 175–185 (2020).
Stampfer, M. J. et al. Test of the National Death Index. Am. J. Epidemiol. 119, 837–839 (1984).
Rich-Edwards, J. W., Corsano, K. A. & Stampfer, M. J. Test of the National Death Index and Equifax Nationwide Death Search. Am. J. Epidemiol. 140, 1016–1019 (1994).
Colditz, G. A. et al. Validation of questionnaire information on risk factors and disease outcomes in a prospective cohort study of women. Am. J. Epidemiol. 123, 894–900 (1986).
Acknowledgements
We thank members of the Vander Heiden laboratory for useful discussions and experimental advice. E.C.L. was supported by the Damon Runyon Cancer Research Foundation (DRG-2299-17). A.N.L. was a Robert Black Fellow of the Damon Runyon Cancer Research Foundation (DRG-2241-15) and was supported by an NIH Pathway to Independence Award (K99CA234221). Z.L. and K.M.S. were supported by NIH training grant T32GM007287. B.M.W. was supported by the Lustgarten Foundation, the Dana-Farber Cancer Institute Hale Family Center for Pancreatic Cancer Research, the NIH (U01CA210171 and P50CA127003), Stand Up To Cancer, the Pancreatic Cancer Action Network, the Noble Effort Fund, the Wexler Family Fund, Promises for Purple and the Bob Parsons Fund. M.G.V.H. acknowledges support from the Emerald Foundation, the Lustgarten Foundation, a Faculty Scholar grant from the Howard Hughes Medical Institute, Stand Up To Cancer, the MIT Center for Precision Cancer Medicine, the Ludwig Center at MIT and the NIH (R35CA242379, R01CA168653, R01CA201276 and P30CA14051). The Nurses’ Health Study and the Health Professionals Follow-up Study were supported by grants UM1 CA186107, U01 CA176726, P01 CA87969, U01 CA167552 and R03 CA223619 from the NIH. The authors thank all participants and staff of the Nurses’ Health Study and the Health Professionals Follow-Up Study for their contributions to this research. We are grateful for help from the following state cancer registries: Alabama, Arizona, Arkansas, California, Colorado, Connecticut, Delaware, Florida, Georgia, Idaho, Illinois, Indiana, Iowa, Kentucky, Louisiana, Maine, Maryland, Massachusetts, Michigan, Nebraska, New Hampshire, New Jersey, New York, North Carolina, North Dakota, Ohio, Oklahoma, Oregon, Pennsylvania, Rhode Island, South Carolina, Tennessee, Texas, Virginia, Washington and Wyoming. The authors also thank the Channing Division of Network Medicine, Brigham and Women’s Hospital and Harvard Medical School for leading the Nurses’ Health Study and the Harvard T.H. Chan School of Public Health for leading the Health Professionals Follow-Up Study. The authors assume full responsibility for analyses and interpretation of these data.
Author information
Authors and Affiliations
Contributions
E.C.L. and M.G.V.H. conceived the project. E.C.L. performed the experiments and analysed data. A.M.W. and A.N.L. assisted with animal experiments and interpreted data. A.M.W., Z.L. and K.M.S. assisted with fatty acid profiling experiments and interpreted data. Y.Z., C.Y. and B.M.W. performed analyses of patient population data from the Nurses’ Health Study and the Health Professionals Follow-up Study. E.C.L. and M.G.V.H. wrote the manuscript, with input from all authors.
Corresponding author
Ethics declarations
Competing interests
M.G.V.H. is a scientific advisor for Agios Pharmaceuticals, Aeglea Biotherapeutics, iTeos Therapeutics and Auron Therapeutics. B.M.W. declares research funding from Celgene and Eli Lilly and consulting for BioLineRx, Celgene and GRAIL. E.C.L., A.M.W., Y.Z., C.Y., Z.L., A.N.L. and K.M.S. declare no competing interests.
Additional information
Peer review information Nature thanks Valter Longo, Costas Lyssiotis, Stephan Herzig and the other, anonymous, reviewer(s) for their contribution to the peer review of this work .
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Caloric restriction, but not the ketogenic diet, impairs growth of tumor allografts.
a–d, Normalized tumor volumes (a, c) and normalized tumor weights (b, d) of subcutaneous AL1376 PDAC allografts in male mice exposed to CR or a control diet (a, b), or the KD or a control diet (c, d). Tumor volumes and weights normalized to animal body weight are shown. (a, b) Control n = 5 mice, CR n = 4 mice; (c, d) Control n = 5 mice, KD n = 5 mice. e–h, Tumor volumes (e, g) and tumor weights (f, h) of subcutaneous AL1376 PDAC allografts in female mice exposed to CR or a control diet (e, f), or the KD or a control diet (g, h). (e, f) Control n = 8 mice, CR n = 8 mice; (g, h) Control n = 4 mice, KD n = 4 mice. i–l, Tumor volumes (i, k) and tumor weights (j, l) of subcutaneous LGSP non-small cell lung cancer (NSCLC) allografts in male (i, j) and female (k, l) mice exposed to CR or a control diet. (i, j) Control n = 10 mice, CR n = 11 mice; (k, l) Control n = 5 mice, CR n = 5 mice. m–p, Tumor volumes (m, o) and tumor weights (n, p) of subcutaneous LGSP NSCLC allografts in male (m, n) and female (o, p) mice exposed to KD or a control diet. (m, n) Control n = 5 mice, KD n = 5 mice; (o, p) Control n = 3 mice, KD n = 3 mice. Data are presented as box-and-whisker plots displaying median, interquartile range (boxes), and minima and maxima (whiskers) (b, d, f, h, j, l, n, p) or mean ± SEM (a, c, e, g, i, k, m, o). Comparisons were made using a two-tailed Student’s t-test (b, d, f, h, j, l, n, p) or two-way repeated measures analysis of variance (ANOVA) (a, c, e, g, i, k, m, o)
Extended Data Fig. 2 Caloric restriction and the ketogenic diet have distinct effects on whole-body physiology.
a, b, Daily food consumption by weight (a) and daily caloric consumption (b) of the control diet and CR by male and female mice. c, Body weights of male and female mice exposed to CR or a control diet. Male Control n = 10, Male CR n = 11, Female Control n = 5, Female CR n = 5. d, e, Ki67 (d) and cleaved caspase 3 (CC3) (e) immunohistochemical staining in subcutaneous AL1376 tumors from mice exposed to a control or CR diet. n = 3 tumors per group. 5-8 independent fields from each tumor were quantified: Control n = 23, CR n = 18 (d); n = 17 per group (e). f, Weights of gastrocnemius muscle (Gastroc) and white adipose tissue (WAT) in male and female mice exposed to CR or a control diet. Tissue weights normalized to animal body weight are shown. n = 5 per group. g–i, Plasma glucagon (g), FGF21 (h), and corticosterone (i) levels in male mice exposed to CR or a control diet. Control n = 6, CR n = 5. j, k, Daily food consumption by weight (j) and daily caloric consumption (k) of the control diet and KD by male and female mice. Male Control n = 5, Male KD n = 5, Female Control n = 4, Female KD n = 5. l, Body weights of male and female mice exposed to KD or a control diet. Male Control n = 5, Male KD n = 5, Female Control n = 3, Female KD n = 3. m, n, Ki67 (m) and CC3 (n) immunohistochemical staining in subcutaneous AL1376 tumors from mice exposed to a control diet or the KD. n = 3 tumors per group. 5-8 independent fields from each tumor were quantified: n = 24 per group (m, n). o, Weights of Gastroc and WAT in male and female mice exposed to KD or a control diet. Tissue weights normalized to animal body weight are shown. Male Control n = 5, Male KD n = 5, Female Control n = 6, Female KD n = 6. p–r, Plasma glucagon (p), FGF21 (q), and corticosterone (r) levels in male mice exposed to KD or a control diet. (p) n = 4 per group; (q–r) n = 5 per group. Data are presented as mean ± SEM (a–c, f–l, o–r) or box-and-whisker plots displaying median, interquartile range (boxes), and minima and maxima (whiskers) (d, e, m, n). Comparisons were made using a two-tailed Student’s t-test
Extended Data Fig. 3 β-OHB is metabolized by PDAC cells.
a, Concentrations of β-OHB in plasma and tumor interstitial fluid (TIF) from mice implanted with subcutaneous AL1376 tumors exposed to a control diet, CR, or the KD. Plasma Control (CR cohort) n = 8, Plasma CR n = 8, Plasma Control (KD cohort) n = 10, Plasma KD n = 9, TIF Control (CR cohort) n = 16, TIF CR n = 13, TIF Control (KD cohort) n = 5, TIF KD n = 6. b, Doubling times of AL1376 cells cultured in media with or without 5 mM β-OHB. c, Fractional labeling of [M+2] TCA cycle metabolites from AL1376 cells cultured in the presence of 5 mM [U-13C]-β-OHB for 24 h. d, Mass isotopologue distribution of labeled palmitate from AL1376 cells cultured in the presence of 5 mM [U-13C]-β-OHB for 24 h. e, f, The indicated fatty acid levels (e) and MUFA/SFA ratios (f) measured in AL1376 cells cultured in media with or without 5 mM β-OHB for 24 h. Data are presented as box-and-whisker plots displaying median, interquartile range (boxes), and minima and maxima (whiskers) (a) or mean ± SEM (b–f). Unless otherwise indicated, n = 3 biologically independent replicates, and comparisons were made using a two-tailed Student’s t-test
Extended Data Fig. 4 Increased stearoyl-CoA desaturase activity is required for cancer cells to adapt to exogenous lipid limitation.
a, Levels of the indicated fatty acids in lipidated and de-lipidated fetal bovine serum (FBS). b, Doubling times of the specified cells cultured in media containing lipidated versus de-lipidated serum. c, Fold changes in levels of the indicated fatty acids in cells cultured in de-lipidated versus lipidated media for 24 h. d, Relative fatty acid synthesis rates in the indicated cells cultured in lipidated versus de-lipidated media. A one-tailed Student’s t-test was used for comparison between groups. e, f, Representative mass isotopologue distributions (MIDs) of 16:0 (e) and 18:0 (f) from AL1376 cells labeled with [U-13C]-glucose and [U-13C]-glutamine for 24 h in lipidated versus de-lipidated media. g, h, Representative MIDs of 16:1(n-7) (g) and 18:1(n-9) (h) from AL1376 cells labeled with [U-13C]-glucose and [U-13C]-glutamine for 24 h in lipidated versus de-lipidated media containing 50 nM A939572. MIDs above M+12 are predominantly observed, consistent with a majority of lipogenic acetyl-CoA being derived from glucose and glutamine. i, Immunoblot for the mature activated form of SREBP1 (m-SREBP1) and β-actin in lysates from AL1376 cells cultured in lipidated versus de-lipidated media for 24 h, with or without 50 nM A939572, 50 µM 18:1(n-9), or 50 µM 18:2(n-6). Data is representative of three biologically independent experiments. j, Absolute fatty acid concentrations in AL1376 and LGSP cells cultured in lipidated media. k, l, Ratios of 16:1(n-7)/16:0 (k) and 18:1(n-9)/18:0 (l) in cells cultured in lipidated versus de-lipidated media with or without the SCD inhibitor A939572 (LGSP: 50 nM; HeLa, Panc1, A549: 200 nM) for 24 h. m, n, 16:1(n-7) (m) and 18:1(n-9) (n) synthesis rates in cells cultured in lipidated versus de-lipidated media with or without A939572 (LGSP: 50 nM; HeLa, Panc1, A549: 200 nM) for 24 h as indicated. A one-tailed ratio paired t-test was used for comparison between groups. o, Doubling times of the specified cells cultured in lipidated versus de-lipidated media with the indicated concentrations of A939572. p, Doubling times of LGSP cells cultured in de-lipidated media with or without 50 nM A939572, BSA, 100 µM 18:1(n-9), 100 µM 18:2(n-6), or 15 µM 16:0. q, Doubling times of cells cultured in de-lipidated media containing 200 nM A939572, BSA, or 25 µM 18:2(n-6). r–v, 18:1(n-9) (r), 16:1(n-7) (s), 18:1(n-9)/18:0 (t), 16:1(n-7)/16:0 (u), and 16:0 (v) levels in LGSP cells cultured in de-lipidated media with or without 50 nM A939572, BSA, 100 µM 18:1(n-9), 100 µM 18:2(n-6), or 15 µM 16:0 for 48 h. w, Relative fatty acid synthesis rates in LGSP cells cultured in de-lipidated media containing 50 nM A939572, BSA, 100 µM 18:1(n-9), or 100 µM 18:2(n-6). x, Doubling times of cells cultured in de-lipidated media containing A939572 (LGSP: 50 nM; HeLa, Panc1, A549: 200 nM), the FASN inhibitor GSK2194069 (LGSP: 0.3 µM; HeLa, A549: 0.015 µM; Panc1: 0.05 µM), or the ACC inhibitor PF-05175157 (LGSP: 10 µM; HeLa: 0.3 µM; Panc1: 1 µM; A549: 0.5 µM). A paired two-tailed Student’s t-test was used for comparison between groups in Panc1 and A549 cells. All data are presented as mean ± SEM; unless otherwise indicated, n = 3 biologically independent replicates, and a two-tailed Student’s t-test was used for comparison between groups unless otherwise noted above
Extended Data Fig. 5 Determining whether SCD inhibition affects cancer cell proliferation via NAD+ limitation or changes to cellular fatty acid levels.
a, Doubling times of the indicated cell lines cultured in de-lipidated media with or without 1 mM pyruvate and rotenone (AL1376: 400 nM; LGSP, A549: 100 nM; HeLa, Panc1: 50 nM). b, Doubling times of the indicated cell lines cultured in de-lipidated media containing the indicated concentrations of the SCD inhibitor A939572 and 1 mM pyruvate. c, Doubling times of the indicated cell lines cultured in de-lipidated media with or without 50 nM A939572. d–i, Relative levels of 16:0 (d), 18:0 (e), 16:1(n-7) (f), 18:1(n-9) (g), 16:1(n-7)/16:0 (h), and 18:1(n-9)/18:0 (i) in the indicated cell lines cultured in de-lipidated media with or without 50 nM A939572. j–l, Relative levels of 16:1(n-10) (j), normalized levels of 16:1(n-10)/16:0 (k), and relative levels of 16:1(n-10)/16:0 (l) in the indicated cell lines cultured in de-lipidated media with or without 50 nM A939572. All data are presented as mean ± SEM; n = 3 biologically independent replicates. A two-tailed Student’s t-test was used for comparison between groups
Extended Data Fig. 6 Caloric restriction and the ketogenic diet alter fatty acid composition and SCD activity in tumors.
a, The indicated fatty acid levels measured in subcutaneous AL1376 tumors from mice exposed to a control or CR diet. Control n = 10, CR n = 8. b–d, The indicated fatty acid levels (b, d) and MUFA/SFA ratios (c) measured in subcutaneous AL1376 tumors from male or female mice exposed to a control or CR diet. Male Control n = 6, Male CR n = 4, Female Control n = 4, Female CR n = 4. e–g, The indicated fatty acid levels (e, g) and MUFA/SFA ratios (f) measured in subcutaneous LGSP tumors from male mice exposed to a control or CR diet. Control n = 6, CR n = 6. h-i, Immunoblot and quantification for mouse Scd1 (mSCD1) and vinculin in subcutaneous AL1376 tumors from female mice (h, n = 7 per group) or subcutaneous LGSP tumors from male mice (i, n = 6 per group) exposed to a control or CR diet. j, The indicated fatty acid levels measured in subcutaneous AL1376 tumors from mice exposed to a control diet or KD. Control n = 8, KD n = 9. k–m, The indicated fatty acid levels (k, m) and MUFA/SFA ratios (l) measured in subcutaneous AL1376 tumors from male or female mice exposed to a control diet or KD. Male Control n = 5, Male KD n = 5, Female Control n = 3, Female KD n = 4. n–p, The indicated fatty acid levels (n, p) and MUFA/SFA ratios (o) measured in subcutaneous LGSP tumors from male mice exposed to a control diet or KD. Control n = 6, KD n = 6. q–r, Immunoblot and quantification for mSCD1 and vinculin in subcutaneous AL1376 tumors from female mice (q, Control n = 3, KD n = 4) or subcutaneous LGSP tumors from male mice (r, n = 6 per group) exposed to a control diet or KD. s, AL1376 and LGSP cells were serum-starved in the presence of 1% Lipid Mixture for 16 h followed by insulin stimulation (100 nM) for 3 h. Lysates were immunoblotted for mSCD1, p-AKT S473, AKT, and vinculin. Data is representative of three biologically independent experiments. Data are presented as box-and-whisker plots displaying median, interquartile range (boxes), and minima and maxima (whiskers) (a, e–g, j, n–p) or mean ± SEM (b–d, h, i, k–m, q–r). Comparisons were made using a two-tailed Student’s t-test
Extended Data Fig. 7 Exogenous SCD expression partially rescues tumor growth inhibition by caloric restriction.
a, b, Tumor volumes (a) and tumor weights (b) normalized to animal body weight of subcutaneous AL1376 tumors expressing EV or SCD-HA in male mice exposed to a control or CR diet. EV: Control n = 4 mice, EV: CR n = 4 mice, SCD-HA: Control n = 4 mice, SCD-HA: CR n = 4 mice. c, d, Tumor volumes (c) and tumor weights (d) of subcutaneous AL1376 tumors expressing EV or SCD-HA in female mice exposed to a control or CR diet. EV: Control n = 4 mice, EV: CR n = 4 mice, SCD-HA: Control n = 4 mice, SCD-HA: CR n = 4 mice. e, f, Tumor volumes (e) and tumor weights (f) normalized to animal body weight of subcutaneous AL1376 tumors expressing EV or SCD-HA in female mice exposed to a control or CR diet. EV: Control n = 4 mice, EV: CR n = 4 mice, SCD-HA: Control n = 4 mice, SCD-HA: CR n = 4 mice. g, h, Daily food consumption by weight (g) and daily caloric consumption (h) of the control diet and CR by male and female mice. i, j, Tumor weights (i) and tumor weights normalized to animal body weight (j) of subcutaneous LGSP tumors expressing EV or SCD-HA in mice exposed to a control or CR diet. EV: Control n = 6 mice, EV: CR n = 5 mice, SCD-HA: Control n = 6 mice, SCD-HA: CR n = 6 mice. k, Immunoblot for HA, human SCD (hSCD), mouse Scd1 (mSCD1), and vinculin in subcutaneous AL1376 tumors expressing an empty vector (EV) or SCD-HA from mice exposed to a control or CR diet for 18 days. l–n, The indicated MUFA/SFA ratios (l) and fatty acid levels (m, n) measured in subcutaneous AL1376 tumors expressing EV or SCD-HA from mice exposed to a control or CR diet for 18 days. EV: Control n = 6, EV: CR n = 6, SCD-HA: Control n = 6, SCD-HA: CR n = 6. o, Immunoblot for HA, hSCD, mSCD1, and vinculin in subcutaneous AL1376 tumors expressing an empty vector (EV) or SCD-HA from mice exposed to a control or CR diet for 7 days. p–r, The indicated MUFA/SFA ratios (p) and fatty acid levels (q–r) measured in subcutaneous AL1376 tumors expressing EV or SCD-HA from mice exposed to a control or CR diet for 7 days. EV: Control n = 6, EV: CR n = 6, SCD-HA: Control n = 6, SCD-HA: CR n = 6. Data are presented as box-and-whisker plots displaying median, interquartile range (boxes), and minima and maxima (whiskers) (b, d, f, i, j, l–n, p–r) or mean ± SEM (a, c, e, g, h). Comparisons were made using two-way repeated measures analysis of variance (ANOVA) (a, c, e) or a two-tailed Student’s t-test (b, d, f, i, j, l–n, p–r)
Extended Data Fig. 8 Changing the fat composition of caloric restriction and the ketogenic diet alters tumor growth and tumor fatty acid composition.
a, b, The indicated fatty acid levels measured in plasma from mice exposed to a control diet, CR, high fat caloric restriction diet consisting of soybean oil (HFCR-Soybean), and high fat caloric restriction diet consisting of palm oil (HFCR-Palm). Control n = 6, CR n = 5, HFCR-Soybean n = 5, HFCR-Palm n = 5. c–e, The indicated fatty acid levels (c, e) and MUFA/SFA ratios (d) measured in subcutaneous AL1376 tumors from mice exposed to a control diet, CR, HFCR-Soybean, or HFCR-Palm. Control n = 7, CR n = 7, HFCR-Soybean n = 7, HFCR-Palm n = 7. f, g, Tumor volumes (f) and tumor weights (g) of subcutaneous LGSP tumors in mice exposed to a control diet, CR, HFCR-Soybean, or HFCR-Palm. Control n = 5 mice, CR n = 6 mice, HFCR-Soybean n = 5 mice, HFCR-Palm n = 6 mice. h–j, The indicated fatty acid levels (h, j) and MUFA/SFA ratios (i) measured in subcutaneous LGSP tumors from mice exposed to a control diet, CR, HFCR-Soybean, or HFCR-Palm. Control n = 6, CR n = 6, HFCR-Soybean n = 6, HFCR-Palm n = 6. k, Tumor weights of subcutaneous LGSP tumors expressing an empty vector (EV) or SCD-HA in mice exposed to a control or KD diet consisting of palm oil (KD-Palm). EV: Control n = 6 mice, EV: KD-Palm n = 6 mice, SCD-HA: Control n = 6 mice, SCD-HA: KD-Palm n = 6 mice. l–n, The indicated fatty acid levels measured in plasma from mice exposed to a control diet or KD-Palm. Control n = 12, KD-Palm n = 12. o–r, The indicated fatty acid levels (o, p, r) and MUFA/SFA ratios (q) measured in subcutaneous AL1376 tumors expressing an EV or SCD-HA from mice exposed to a control diet or KD-Palm. EV: Control n = 6, EV: KD-Palm n = 7, SCD-HA: Control n = 6, SCD-HA: KD-Palm n = 6. Data are presented as box-and-whisker plots displaying median, interquartile range (boxes), and minima and maxima (whiskers) (a–e, g–r) or mean ± SEM (f). Comparisons were made using a two-tailed Student’s t-test (a–e, h–r), a two-way repeated measures analysis of variance (ANOVA) (f), or a one-way ANOVA (g)
Supplementary information
Supplementary Figs.
Supplementary Figs. 1–23, containing uncropped scans of western blots.
Supplementary Table 1
Raw data used for fatty acid source analysis (FASA) synthesis rate calculations in Fig. 3 and Extended Data Fig. 4 .
Source data
Rights and permissions
About this article
Cite this article
Lien, E.C., Westermark, A.M., Zhang, Y. et al. Low glycaemic diets alter lipid metabolism to influence tumour growth. Nature 599, 302–307 (2021). https://doi.org/10.1038/s41586-021-04049-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-04049-2
This article is cited by
-
Lipids as mediators of cancer progression and metastasis
Nature Cancer (2024)
-
A Machine Learning Computational Framework Develops a Multiple Programmed Cell Death Index for Improving Clinical Outcomes in Bladder Cancer
Biochemical Genetics (2024)
-
Energy metabolism as the hub of advanced non-small cell lung cancer management: a comprehensive view in the framework of predictive, preventive, and personalized medicine
EPMA Journal (2024)
-
Is metabolism the magic bullet for targeted cancer therapy?
BMC Cancer (2023)
-
Acetyl-CoA metabolism in cancer
Nature Reviews Cancer (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.