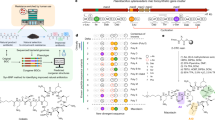
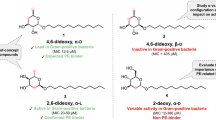
Abstract
The development of new antibiotics to treat infections caused by drug-resistant Gram-negative pathogens is of paramount importance as antibiotic resistance continues to increase worldwide1. Here we describe a strategy for the rational design of diazabicyclooctane inhibitors of penicillin-binding proteins from Gram-negative bacteria to overcome multiple mechanisms of resistance, including β-lactamase enzymes, stringent response and outer membrane permeation. Diazabicyclooctane inhibitors retain activity in the presence of β-lactamases, the primary resistance mechanism associated with β-lactam therapy in Gram-negative bacteria2,3. Although the target spectrum of an initial lead was successfully re-engineered to gain in vivo efficacy, its ability to permeate across bacterial outer membranes was insufficient for further development. Notably, the features that enhanced target potency were found to preclude compound uptake. An improved optimization strategy leveraged porin permeation properties concomitant with biochemical potency in the lead-optimization stage. This resulted in ETX0462, which has potent in vitro and in vivo activity against Pseudomonas aeruginosa plus all other Gram-negative ESKAPE pathogens, Stenotrophomonas maltophilia and biothreat pathogens. These attributes, along with a favourable preclinical safety profile, hold promise for the successful clinical development of the first novel Gram-negative chemotype to treat life-threatening antibiotic-resistant infections in more than 25 years.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
Atomic coordinates and structure factors of the PaPBP3–ETX0462 structure have been deposited in the Protein Data Bank under accession code 7JWL. Source data are provided with this paper.
References
Tacconelli, E. et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 18, 318–327 (2018).
Bonomo, R. A. Beta-lactamases: a focus on current challenges. Cold Spring Harb. Perspect. Med. 7, a025239 (2016).
Bush, K. & Bradford, P. A. Interplay between β-lactamases and new β-lactamase inhibitors. Nat. Rev. Microbiol. 17, 295–306 (2019).
Ehmann, D. E. et al. Avibactam is a covalent, reversible, non-β-lactam β-lactamase inhibitor. Proc. Natl Acad. Sci. USA 109, 11663–11668 (2012).
Livermore, D. M., Mushtaq, S., Warner, M., Vickers, A. & Woodford, N. In vitro activity of cefepime/zidebactam (WCK 5222) against Gram-negative bacteria. J. Antimicrob. Chemother. 72, 1373–1385 (2017).
Morinaka, A. et al. OP0595, a new diazabicyclooctane: mode of action as a serine β-lactamase inhibitor, antibiotic and β-lactam 'enhancer'. J. Antimicrob. Chemother. 70, 2779–2786 (2015).
Durand-Reville, T. F. et al. ETX2514 is a broad-spectrum β-lactamase inhibitor for the treatment of drug-resistant Gram-negative bacteria including Acinetobacter baumannii. Nat. Microbiol. 2, 17104 (2017).
Miller, A. A. et al. In vitro characterization of ETX1317, a broad-spectrum β-lactamase inhibitor that restores and enhances β-lactam activity against multi-drug-resistant Enterobacterales, including carbapenem-resistant strains. ACS Infect. Dis. 6, 1389–1397 (2020).
Wang, D. Y., Abboud, M. I., Markoulides, M. S., Brem, J. & Schofield, C. J. The road to avibactam: the first clinically useful non-β-lactam working somewhat like a β-lactam. Future Med. Chem. 8, 1063–1084 (2016).
Levy, N. et al. Structural basis for E. coli penicillin binding protein (PBP) 2 inhibition, a platform for drug design. J. Med. Chem. 62, 4742–4754 (2019).
Kidd, J. M., Abdelraouf, K. & Nicolau, D. P. Efficacy of human-simulated bronchopulmonary exposures of cefepime, zidebactam and the combination (WCK 5222) against MDR Pseudomonas aeruginosa in a neutropenic murine pneumonia model. J. Antimicrob. Chemother. 75, 149–155 (2020).
O'Donnell, J. et al. Pharmacokinetic/pharmacodynamic determination and preclinical pharmacokinetics of the β-lactamase inhibitor ETX1317 and its orally available prodrug ETX0282. ACS Infect. Dis. 6, 1378–1388 (2020).
Doumith, M., Mushtaq, S., Livermore, D. M. & Woodford, N. New insights into the regulatory pathways associated with the activation of the stringent response in bacterial resistance to the PBP2-targeted antibiotics, mecillinam and OP0595/RG6080. J. Antimicrob. Chemother. 71, 2810–2814 (2016).
Levasseur, P., Pace, J., Lee Coleman, K. & Lowther, J. Novel Combinations of Antibacterial Nitrogenous Heterocyclic Compounds with Other Antibacterial Compounds, and Use Thereof as Drugs. Patent Cooperation Treaty WO/2010/041112 (2010).
Aszodi, J., Lampilas, M., Musicki, B., Rowlands, D. A. & Colette, P. Heterocyclic Compounds, Method for Preparing Same and Use Thereof as Medicines, in Particular as Antibacterial Agents. Patent Cooperation Treaty WO/2002/100860 (2001).
Nonejuie, P., Burkart, M., Pogliano, K. & Pogliano, J. Bacterial cytological profiling rapidly identifies the cellular pathways targeted by antibacterial molecules. Proc. Natl Acad. Sci. USA 110, 16169–16174 (2013).
Lomovskaya, O. et al. Identification and characterization of inhibitors of multidrug resistance efflux pumps in Pseudomonas aeruginosa: novel agents for combination therapy. Antimicrob. Agents Chemother. 45, 105–116 (2001).
Viljanen, P. & Vaara, M. Susceptibility of Gram-negative bacteria to polymyxin B nonapeptide. Antimicrob. Agents Chemother. 25, 701–705 (1984).
Iyer, R. et al. Whole-cell-based assay to evaluate structure permeation relationships for carbapenem passage through the Pseudomonas aeruginosa porin OprD. ACS Infect. Dis. 3, 310–319 (2017).
Chevalier, S. et al. Structure, function and regulation of Pseudomonas aeruginosa porins. FEMS Microbiol. Rev. 41, 698–722 (2017).
Hancock, R. E. & Brinkman, F. S. Function of Pseudomonas porins in uptake and efflux. Ann. Rev. Microbiol. 56, 17–38 (2002).
Nikaido, H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol. Mol. Biol. Rev. 67, 593–656 (2003).
Kos, V. N. et al. The resistome of Pseudomonas aeruginosa in relationship to phenotypic susceptibility. Antimicrob. Agents Chemother. 59, 427–436 (2015).
Dötsch, A. et al. The Pseudomonas aeruginosa transcriptome in planktonic cultures and static biofilms using RNA sequencing. PLoS ONE 7, e31092 (2012).
Turner, K. H., Everett, J., Trivedi, U., Rumbaugh, K. P. & Whiteley, M. Requirements for Pseudomonas aeruginosa acute burn and chronic surgical wound infection. PLoS Genet. 10, e1004518 (2014).
Richter, M. F. et al. Predictive compound accumulation rules yield a broad-spectrum antibiotic. Nature 545, 299–304 (2017).
Eren, E. et al. Substrate specificity within a family of outer membrane carboxylate channels. PLoS Biol. 10, e1001242 (2012).
Bajaj, H. et al. Bacterial outer membrane porins as electrostatic nanosieves: exploring transport rules of small polar molecules. ACS Nano 11, 5465–5473 (2017).
Cadwell, J. J. S. The hollow fiber infection model for antimicrobial pharmacodynamics and pharmacokinetics. Adv. Pharmacoepidem. Drug Safety S1, 1–5 (2012).
Sastry, G. M., Adzhigirey, M., Day, T., Annabhimoju, R. & Sherman, W. Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J. Comp. Aid. Mol. Des. 27, 221–234 (2013).
Hess, B., Kutzner, C., van der Spoel, D. & Lindahl, E. GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theor. Comput. 4, 435–447 (2008).
Humphrey, W., Dalke, A. & Schulten, K. VMD: visual molecular dynamics. J. Mol. Graph. 14, 33–38 (1996).
Sindhikara, D. J., Yoshida, N. & Hirata, F. Placevent: an algorithm for prediction of explicit solvent atom distribution-application to HIV-1 protease and F-ATP synthase. J. Comp. Chem. 33, 1536–1543 (2012).
Velez-Vega, C., McKay, D. J., Aravamuthan, V., Pearlstein, R. & Duca, J. S. Time-averaged distributions of solute and solvent motions: exploring proton wires of GFP and PfM2DH. J. Chem. Inform. Model. 54, 3344–3361 (2014).
M07: Methods for Dilution Antimicrobial Susceptibilty Tests for Bacteria that Grow Aerobically 11th edn (Clinical and Laboratory Standards Institute, 2018).
M26: Methods for Determining Bactericidal Activity 19th edn (Clinical and Laboratory Standards Institute, 1999).
Iyer, R., Moussa, S. H., Durand-Réville, T. F., Tommasi, R. & Miller, A. Acinetobacter baumannii OmpA is a selective antibiotic permeant porin. ACS Infect. Dis. 4, 373–381 (2018).
Penwell, W. F. et al. Molecular mechanisms of sulbactam antibacterial activity and resistance determinants in Acinetobacter baumannii. Antimicrob. Agents Chemother. 59, 1680–1689 (2015).
Shapiro, A. B., Gao, N., Gu, R. F. & Thresher, J. Fluorescence anisotropy-based measurement of Pseudomonas aeruginosa penicillin-binding protein 2 transpeptidase inhibitor acylation rate constants. Anal. Biochem. 463, 15–22 (2014).
Shapiro, A. B., Gu, R. F., Gao, N., Livchak, S. & Thresher, J. Continuous fluorescence anisotropy-based assay of BOCILLIN FL penicillin reaction with penicillin binding protein 3. Anal. Biochem. 439, 37–43 (2013).
Murphy-Benenato, K. E. et al. SAR and structural analysis of siderophore-conjugated monocarbam inhibitors of PBP3. ACS Med. Chem. Lett. 6, 537–542 (2015).
Adams, P. D. et al. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D 66, 213–221 (2010).
Gerber, A. U. et al. Impact of dosing intervals on activity of gentamicin and ticarcillin against Pseudomonas aeruginosa in granulocytopenic mice. J. Infect. Dis. 147, 910–917 (1983).
Gudmundsson, S., Vogelman, B. & Craig, W. A. The in-vivo postantibiotic effect of imipenem and other new antimicrobials. J. Antimicrob. Chemother. 18, 67–73 (1986).
Goutelle, S. et al. The Hill equation: a review of its capabilities in pharmacological modelling. Fundam. Clin. Pharmacol. 22, 633–648 (2008).
Institute of Laboratory Animal Resources Commission of Life Sciences. A Guide for the Care and Use of Laboratory Animals (National Academy Press, 1996).
Byrne, W. R. et al. Antibiotic treatment of experimental pneumonic plague in mice. Antimicrob. Agents Chemother. 42, 675–681 (1998).
May, K. R. The Collison nebulizer: description, performance and application. J. Aerosol Sci. 4, 235–243 (1973).
Hartings, J. M. & Roy, C. J. The automated bioaerosol exposure system: preclinical platform development and a respiratory dosimetry application with nonhuman primates. J. Pharmacol. Toxicol. Methods 49, 39–55 (2004).
Guyton, A. C. Measurement of the respiratory volumes of laboratory animals. Am. J. Physiol. 150, 70–77 (1947).
Morris, J. et al. Neurotropic threat characterization of Burkholderia pseudomallei strains. Emerg. Infect Dis. 21, 58–63 (2015).
Acknowledgements
We thank D. McKinney, K. Thakur, S. Narayan and Pharmaron for their chemistry support; IHMA and JMI laboratories for microbiological surveillance studies; Neosome Life Sciences for in vivo efficacy studies; MPI Research for preclinical toxicology studies and J. Abendroth for help with crystallography. This research programme was partially supported by the Cooperative Agreement No. 4500002444 from ASPR/BARDA and by awards from Wellcome Trust and Germany’s Federal Ministry of Education and Research (BMBF), as administrated by CARB-X. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Department of Health and Human Services Office of the Assistant Secretary for Preparedness and Response, other funders, or CARB-X. This research used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract No. DE-AC02-06CH11357. Use of the LS-CAT Sector 21 was supported by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor (grant 085P1000817).
Author information
Authors and Affiliations
Contributions
R.A.T. oversaw execution of all aspects of the project. T.F.D.-R., A.A.M. and J.P.O. wrote the manuscript and directed chemistry, biology and drug metabolism and pharmacokinetics/toxicology studies, respectively. X.W., M.A.S., S.G., J.Z., J.C.-P., J.A.R. and H.H. performed medicinal chemistry experiments. N.M.C., S.M.M., S.H.M. and R.I. performed microbiological experiments. A.C. and A.M.T. performed drug metabolism and pharmacokinetics experiments. S.H.M. performed microscopy, whole-genome sequencing and analysis. R.I. performed TOMAS. A.B.S. performed biochemical experiments. C.V.-V. generated and analysed MD simulations. A.D.F., P.S.H. and S.J.M. performed crystallography experiments. H.S.H., G.L.D., J.E.C. and R.A.S. performed microbiological and in vivo efficacy experiments using biothreat pathogens.
Corresponding author
Ethics declarations
Competing interests
T.F.D.-R., A.A.M., J.P.O., X.W., M.A.S., S.G., R.I., A.B.S., N.M.C., C.V.-V., S.H.M., S.M.M., A.C., A.M.T., J.Z., J.C.-P., J.A.R. and R.A.T. are current or former employees of Entasis Therapeutics. H.H., A.D.F., P.S.H., S.J.M., H.S.H., G.L.D., J.E.C. and R.A.S. have no competing interests to declare.
Additional information
Peer review information Nature thanks the anonymous reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Compound-dependent bacterial morphologies and evidence of porin overexpression in TOMAS.
a, Morphology of P. aeruginosa PAO1 without drug treatment (left panel, 5 µm scale bar) or treated with 0.5X MIC of either NXL-105 (middle panel, 5 µm scale bar) or Compound 2 (right panel, 10 µm scale bar). Micrographs are representative of results obtained for three biologically independent studies. b, SDS-PAGE analysis of overexpressed porins in TOMAS19 after overnight induction with 120 µM L-arabinose. 2 µg outer membrane extracts were loaded onto a 13% polyacrylamide-SDS gel in 6 M urea. Expression levels are comparable to those observed for OprD in TOMAS19. Gel is representative of two biologically independent studies. Bands corresponding to the overexpressed porins are highlighted in boxes. Upper panel = P. aeruginosa porins; lower panel = K. pneumoniae porins.
Extended Data Fig. 2 In vitro and in vivo activity of ETX0462.
a, Percent cumulative growth inhibition of 40 MDR P. aeruginosa clinical isolates from the CDC MDR P. aeruginosa panel at increasing concentrations of ETX0462 (blue), meropenem-vaborbactam (MEM-VAB, red), imipenem-relebactam (IMI-REL, orange, ceftolozane-tazobactam (TOL-TAZ, purple) and ceftazidime-avibactam (CAZ-AVI, green). MICs were measured in singleton. b, A composite Emax fitting of the %fT>MIC vs change in CFU burden for 7 strains (MIC range of 0.25 − 4 mg/L, R2 = 0.87) evaluated in a murine neutropenic thigh model in vivo suggests > 1-log kill when unbound systemic concentrations of ETX0462 exceed the MIC for 60% of the dosing interval. c, Kaplan-Meier survival plot of Y. pestis CO92 infected mice using an aerosolized dose (20 X LD50 of 6.8 x 104 CFU) followed by treatment with ETX0462 vs. vehicle control, ciprofloxacin (CIP) or ceftazidime (CTZ) shows equivalent in vivo efficacy (Log-rank test p < 0.0001 vs. control).
Supplementary information
Supplementary Information
This file contains Supplementary Methods, details regarding the synthetic scheme and experimental procedure for ETX0462 and compound 2, HPLC and NMR spectra data, Tables 1, 2 and Supplementary Fig. 1.
Rights and permissions
About this article
Cite this article
Durand-Reville, T.F., Miller, A.A., O’Donnell, J.P. et al. Rational design of a new antibiotic class for drug-resistant infections. Nature 597, 698–702 (2021). https://doi.org/10.1038/s41586-021-03899-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03899-0
This article is cited by
-
Discovery of a structural class of antibiotics with explainable deep learning
Nature (2024)
-
A pH/enzyme dual responsive PMB spatiotemporal release hydrogel promoting chronic wound repair
Journal of Nanobiotechnology (2023)
-
Porin-independent accumulation in Pseudomonas enables antibiotic discovery
Nature (2023)
-
Organocatalytic atroposelective construction of axially chiral N, N- and N, S-1,2-azoles through novel ring formation approach
Nature Communications (2022)
-
AB-DB: Force-Field parameters, MD trajectories, QM-based data, and Descriptors of Antimicrobials
Scientific Data (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.