Abstract
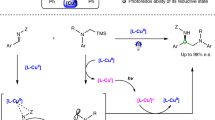
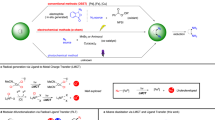
The substitution of an alkyl electrophile by a nucleophile is a foundational reaction in organic chemistry that enables the efficient and convergent synthesis of organic molecules. Although there has been substantial recent progress in exploiting transition-metal catalysis to expand the scope of nucleophilic substitution reactions to include carbon nucleophiles1,2,3,4, there has been limited progress in corresponding reactions with nitrogen nucleophiles5,6,7,8. For many substitution reactions, the bond construction itself is not the only challenge, as there is a need to control stereochemistry at the same time. Here we describe a method for the enantioconvergent substitution of unactivated racemic alkyl electrophiles by a ubiquitous nitrogen-containing functional group, an amide. Our method uses a photoinduced catalyst system based on copper, an Earth-abundant metal. This process for asymmetric N-alkylation relies on three distinct ligands—a bisphosphine, a phenoxide and a chiral diamine. The ligands assemble in situ to form two distinct catalysts that act cooperatively: a copper/bisphosphine/phenoxide complex that serves as a photocatalyst, and a chiral copper/diamine complex that catalyses enantioselective C–N bond formation. Our study thus expands enantioselective N-substitution by alkyl electrophiles beyond activated electrophiles (those bearing at least one sp- or sp2-hybridized substituent on the carbon undergoing substitution)8,9,10,11,12,13 to include unactivated electrophiles.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available within the paper, its Supplementary Information (experimental procedures and characterization data) and from the Cambridge Crystallographic Data Centre (https://www.ccdc.cam.ac.uk/structures; crystallographic data are available free of charge under CCDC reference numbers CCDC 2055329–2055338).
References
Fu, G. C. Transition-metal catalysis of nucleophilic substitution reactions: a radical alternative to SN1 and SN2 processes. ACS Cent. Sci. 3, 692–700 (2017).
Choi, J. & Fu, G. C. Transition metal-catalyzed alkyl–alkyl bond formation: another dimension in cross-coupling chemistry. Science 356, eaaf7230 (2017).
Kaga, A. & Chiba, S. Engaging radicals in transition metal-catalyzed cross-coupling with alkyl electrophiles: recent advances. ACS Catal. 7, 4697–4706 (2017).
Iwasaki, T. & Kambe, N. Ni-catalyzed C–C couplings using alkyl electrophiles. Top. Curr. Chem. 374, 66 (2016).
Bissember, A. C., Lundgren, R. J., Creutz, S. E., Peters, J. C. & Fu, G. C. Transition-metal-catalyzed alkylations of amines with alkyl halides: photoinduced, copper-catalyzed couplings of carbazoles. Angew. Chem. Int. Ed. 52, 5129–5133 (2013).
Do, H.-Q., Bachman, S., Bissember, A. C., Peters, J. C. & Fu, G. C. Photoinduced, copper-catalyzed alkylation of amides with unactivated secondary alkyl halides at room temperature. J. Am. Chem. Soc. 136, 2162–2167 (2014).
Peacock, D. M., Roos, C. B. & Hartwig, J. F. Palladium-catalyzed cross coupling of secondary and tertiary alkyl bromides with a nitrogen nucleophile. ACS Cent. Sci. 2, 647–652 (2016).
Grange, R. L., Clizbe, E. A. & Evans, P. A. Recent developments in asymmetric allylic amination reactions. Synthesis 48, 2911–2968 (2016).
Zhang, H. et al. Construction of the N1−C3 linkage stereogenic centers by catalytic asymmetric amination reaction of 3-bromooxindoles with indolines. Org. Lett. 16, 2394–2397 (2014).
Kainz, Q. M. et al. Asymmetric copper-catalyzed C–N cross-couplings induced by visible light. Science 351, 681–684 (2016).
Zhang, X. et al. An enantioconvergent halogenophilic nucleophilic substitution (SN2X) reaction. Science 363, 400–404 (2019).
Bartoszewicz, A., Matier, C. D. & Fu, G. C. Enantioconvergent alkylations of amines by alkyl electrophiles: copper-catalyzed nucleophilic substitutions of racemic α-halolactams by indoles. J. Am. Chem. Soc. 141, 14864–14869 (2019).
Wang, Y., Wang, S., Shan, W. & Shao, Z. Direct asymmetric N-propargylation of indoles and carbazoles catalyzed by lithium SPINOL phosphate. Nat. Commun. 11, 226 (2020).
Greenberg, A., Breneman, C. M. & Liebman, J. F. (eds). The Amide Linkage: Structural Significance in Chemistry, Biochemistry, and Materials Science (Wiley, 2000).
Ponra, S., Boudet, B., Phansavath, P. & Ratovelomanana-Vidal, V. Recent developments in transition-metal-catalyzed asymmetric hydrogenation of enamides. Synthesis 53, 193–214 (2021).
Wang, J.-W. et al. Catalytic asymmetric reductive hydroalkylation of enamides and enecarbamates to chiral aliphatic amines. Nat. Commun. 12, 1313 (2020).
Brown, D. G. & Böstrom, J. Analysis of past and present synthetic methodologies on medicinal chemistry: where have all the new reactions gone? J. Med. Chem. 59, 4443–4458 (2016).
Taylor, J. E. & Bull, S. D. in Comprehensive Organic Synthesis 2nd edn, vol. 6 (eds Knochel, P. & Molander, G. A.) 427–478 (Elsevier, 2014).
Carey, J. S., Laffan, D., Thomson, C. & Williams, M. T. Analysis of the reactions used for the preparation of drug candidate molecules. Org. Biomol. Chem. 4, 2337–2347 (2006).
Matier, C. D., Schwaben, J., Peters, J. C. & Fu, G. C. Copper-catalyzed alkylation of aliphatic amines induced by visible light. J. Am. Chem. Soc. 139, 17707–17710 (2017).
Ahn, J. M., Peters, J. C. & Fu, G. C. Design of a photoredox catalyst that enables the direct synthesis of carbamate-protected primary amines via photoinduced, copper-catalyzed N-alkylation reactions of unactivated secondary halides. J. Am. Chem. Soc. 139, 18101–18106 (2017).
Hossain, A., Bhattacharyya, A. & Reiser, O. Copper’s rapid ascent in visible-light photoredox catalysis. Science 364, eaav9713 (2019).
Cheng, L.-J. & Mankad, N. P. C–C and C–X coupling reactions of unactivated alkyl electrophiles using copper catalysis. Chem. Soc. Rev. 49, 8036–8064 (2020).
Carreira, E. M. & Yamamoto, H. (eds.) Comprehensive Chirality (Academic, 2012).
Reyes, R. L. et al. Asymmetric remote C–H borylation of aliphatic amides and esters with a modular iridium catalyst. Science 369, 970–974 (2020).
Ordóñez, M., Labastida-Galvan, V. & Lagunas-Rivera, S. Stereoselective synthesis of GABOB, carnitine and statine phosphonates analogues. Tetrahedron Asymmetry 21, 129–147 (2010).
Hale, J. J. et al. The discovery of 3-(N-alkyl)aminopropylphosphonic acids as potent S1P receptor agonists. Bioorg. Med. Chem. Lett. 14, 3495–3499 (2004).
Ordóñez, M., Cativiela, C. & Romero-Estudillo, I. An update on the stereoselective synthesis of γ-amino acids. Tetrahedron Asymmetry 27, 999–1055 (2016).
Souto, J. A. & Muniz, K. in Asymmetric Synthesis vol. 2 (eds Christmann, M. & Brase, S) 371–375 (Wiley-VCH, 2012).
Antonopoulou, G. et al. Synthesis of 2-oxoamides based on sulfonamide analogs of γ-amino acids and their activity on phospholipase A2. J. Pept. Sci. 14, 1111–1120 (2008).
Comprehensive Asymmetric Catalysis vol. I–III (eds. Jacobsen, E. N., Pfaltz, A. & Yamamoto, H.) (Springer, 1999).
Teegardin, K., Day, J. I., Chan, J. & Weaver, J. Advances in photocatalysis: a microreview of visible light mediated ruthenium and iridium catalyzed organic transformations. Org. Process Res. Dev. 20, 1156–1163 (2016).
Creutz, S. E., Lotito, K. J., Fu, G. C. & Peters, J. C. Photoinduced Ullmann C–N coupling: demonstrating the viability of a radical pathway. Science 338, 647–651 (2012).
Ribelli, T. G., Lorandi, F., Fantin, M. & Matyjaszewski, K. Atom transfer radical polymerization: billion times more active catalysts and new initiation systems. Macromol. Rapid Commun. 40, 1800616 (2019).
Lusztyuk, J., Maillard, B., Deycard, S., Lindsay, D. A. & Ingold, K. U. Kinetics for the reaction of a secondary alkyl radical with tri-n-butylgermanium hydride and calibration of a secondary alkyl radical clock reaction. J. Org. Chem. 52, 3509–3514 (1987).
Anslyn, E. V. & Dougherty, D. A. in Modern Physical Organic Chemistry, 156 (University Science Books, 2006).
Acknowledgements
This manuscript is dedicated to the memory of Gregory P. Harlow. Support has been provided by the National Institutes of Health (National Institute of General Medical Sciences, R01–GM109194), the Beckman Institute (support of the Laser Resource Center, as well as the Center for Catalysis and Chemical Synthesis, the EPR facility, and the X-ray crystallography facility), the Gordon and Betty Moore Foundation (support for the Center for Catalysis and Chemical Synthesis), the Dow Next-Generation Educator Fund (grant to Caltech) and Boehringer–Ingelheim Pharmaceuticals. We thank C. Citek, T. M. Donnell, J. Dørfler, P. Garrido Barros, L. M. Henling, P. H. Oyala, F. Schneck, M. Shahgoli, D. VanderVelde and J. R. Winkler for assistance and discussions.
Author information
Authors and Affiliations
Contributions
C.C. performed all experiments. C.C., J.C.P. and G.C.F. wrote the manuscript. All authors contributed to the analysis and the interpretation of the results.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Continued scope of alkyl bromides serving as electrophilic coupling partners.
Couplings were generally conducted using 0.5 mmol of the amide, and N1* was used as the diamine, unless otherwise noted. All data represent the average of two experiments. The per cent yield represents purified product. aElectrophile (1.5 equiv.), Cu(CH3CN)4PF6 (15 mol%), P (5 mol%), N1* (20 mol%), K3PO4 ∙ H2O (1.5 equiv.; in place of Cs2CO3), 10 °C. Ar1, p-(F3C)C6H4.
Supplementary information
Supplementary Information
This file contains Supplementary Text and Data, Supplementary Tables 1-2, Supplementary Figures 1-31 and NMR Spectra Data - see contents page for details.
Rights and permissions
About this article
Cite this article
Chen, C., Peters, J.C. & Fu, G.C. Photoinduced copper-catalysed asymmetric amidation via ligand cooperativity. Nature 596, 250–256 (2021). https://doi.org/10.1038/s41586-021-03730-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03730-w
This article is cited by
-
Enantioselective propargylic amination and related tandem sequences to α-tertiary ethynylamines and azacycles
Nature Chemistry (2024)
-
Reduction of unactivated alkyl chlorides enabled by light-induced single electron transfer
Science China Chemistry (2024)
-
Enantioconvergent Cu-catalysed N-alkylation of aliphatic amines
Nature (2023)
-
Copper-catalysed enantioconvergent alkylation of oxygen nucleophiles
Nature (2023)
-
Direct access to chiral aliphatic amines by catalytic enantioconvergent redox-neutral amination of alcohols
Nature Synthesis (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.