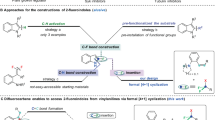
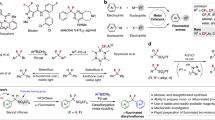
Abstract
Fluoroalkyl groups profoundly affect the physical properties of pharmaceuticals and influence almost all metrics associated with their pharmacokinetic and pharmacodynamic profile1,2,3,4. Drug candidates increasingly contain trifluoromethyl (CF3) and difluoromethyl (CF2H) groups, and the same trend in agrochemical development shows that the effect of fluoroalkylation translates across human, insect and plant life5,6. New fluoroalkylation reactions have undoubtedly stimulated this shift; however, methods that directly convert C–H bonds into C–CF2X groups (where X is F or H) in complex drug-like molecules are rare7,8,9,10,11,12,13. Pyridines are the most common aromatic heterocycles in pharmaceuticals14, but only one approach—via fluoroalkyl radicals—is viable for achieving pyridyl C–H fluoroalkylation in the elaborate structures encountered during drug development15,16,17. Here we develop a set of bench-stable fluoroalkylphosphines that directly convert the C–H bonds in pyridine building blocks, drug-like fragments and pharmaceuticals into fluoroalkyl derivatives. No preinstalled functional groups or directing groups are required. The reaction tolerates a variety of sterically and electronically distinct pyridines, and is exclusively selective for the 4-position in most cases. The reaction proceeds through initial formation of phosphonium salts followed by sp2–sp3 coupling of phosphorus ligands—an underdeveloped manifold for forming C–C bonds.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in the published article (and its Supplementary Information files). The computational chemistry datasets generated and analysed here are available in Zenodo at https://doi.org/10.5281/zenodo.4554587.
Code availability
Computational chemistry datasets were analysed with Goodvibes version 3.0.0, available at Github (https://github.com/bobbypaton/GoodVibes) and Zenodo (https://github.com/bobbypaton/GoodVibes).
References
Müller, K., Faeh, C. & Diederich, F. Fluorine in pharmaceuticals: looking beyond intuition. Science 317, 1881–1886 (2007).
Hagmann, W. K. The many roles for fluorine in medicinal chemistry. J. Med. Chem. 51, 4359–4369 (2008).
Zhou, Y. et al. Next generation of fluorine-containing pharmaceuticals, compounds currently in phase II–III clinical trials of major pharmaceutical companies: new structural trends and therapeutic areas. Chem. Rev. 116, 422–518 (2016).
Zafrani, Y. et al. Difluoromethyl bioisostere: examining the “lipophilic hydrogen bond donor” concept. J. Med. Chem. 60, 797–804 (2017).
Jeschke, P. The unique role of fluorine in the design of active ingredients for modern crop protection. ChemBioChem 5, 570–589 (2004).
Burriss, A. et al. The importance of trifluoromethyl pyridines in crop protection. Pest Manag. Sci. 74, 1228–1238 (2018).
Alonso, C., Martínez de Marigorta, E., Rubiales, G. & Palacios, F. Carbon trifluoromethylation reactions of hydrocarbon derivatives and heteroarenes. Chem. Rev. 115, 1847–1935 (2015).
Barata-Vallejo, S., Lantaño, B. & Postigo, A. Recent advances in trifluoromethylation reactions with electrophilic trifluoromethylating reagents. Chem. Eur. J. 20, 16806–16829 (2014).
Yerien, D. E., Barata-Vallejo, S. & Postigo, A. Difluoromethylation reactions of organic compounds. Chem. Eur. J. 23, 14676–14701 (2017).
Postigo, A. in Late-Stage Fluorination of Bioactive Molecules and Biologically-Relevant Substrates (ed. Postigo, A.) 243–285 (Elsevier, 2019).
Barata-Vallejo, S. & Postigo, A. Photocatalytic difluoromethylation reactions of aromatic compounds and aliphatic multiple C–C bonds. Molecules 24, 4483 (2019).
Lemos, A., Lemaire, C. & Luxen, A. Progress in difluoroalkylation of organic substrates by visible light photoredox catalysis. Adv. Synth. Catal. 361, 1500–1537 (2019).
Ye, F. et al. Aryl sulfonium salts for site-selective late-stage trifluoromethylation. Angew. Chem. Int. Ed. 58, 14615–14619 (2019).
Vitaku, E., Smith, D. T. & Njardarson, J. T. Analysis of the structural diversity, substitution patterns, and frequency of nitrogen heterocycles among U.S. FDA approved pharmaceuticals. J. Med. Chem. 57, 10257–10274 (2014).
Nagib, D. A. & MacMillan, D. W. C. Trifluoromethylation of arenes and heteroarenes by means of photoredox catalysis. Nature 480, 224–228 (2011).
Fujiwara, Y. et al. Practical and innate carbon-hydrogen functionalization of heterocycles. Nature 492, 95–99 (2012).
Beatty, J. W., Douglas, J. J., Cole, K. P. & Stephenson, C. R. J. A scalable and operationally simple radical trifluoromethylation. Nat. Commun. 6, 7919 (2015).
Ekroos, M. & Sjögren, T. Structural basis for ligand promiscuity in cytochrome P450 3A4. Proc. Natl Acad. Sci. USA 103, 13682–13687 (2006).
Gibbs, M. A. & Hosea, N. A. Factors affecting the clinical development of cytochrome P450 3A substrates. Clin. Pharmacokinet. 42, 969–984 (2003).
O’Hara, F., Blackmond, D. G. & Baran, P. S. Radical-based regioselective C–H functionalization of electron-deficient heteroarenes: scope, tunability, and predictability. J. Am. Chem. Soc. 135, 12122–12134 (2013).
Nagase, M., Kuninobu, Y. & Kanai, M. 4-Position-selective C–H perfluoroalkylation and perfluoroarylation of six-membered heteroaromatic compounds. J. Am. Chem. Soc. 138, 6103–6106 (2016).
Finet, J.-P. Ligand Coupling Reactions with Heteroatomic Compounds 1st edn (Pergamon, 1998).
Uchida, Y., Onoue, K., Tada, N. & Nagao, F. Ligand coupling reaction on the phosphorus atom. Tetrahedr. Lett. 30, 567–570 (1989).
Hilton, M. C. et al. Heterobiaryl synthesis by contractive C–C coupling via P(V) intermediates. Science 362, 799–804 (2018).
Boyle, B. T., Hilton, M. C. & McNally, A. Nonsymmetrical bis-azine biaryls from chloroazines: a strategy using phosphorus ligand-coupling. J. Am. Chem. Soc. 141, 15441–15449 (2019).
Krishnamurti, V., Barrett, C. & Prakash, G. K. S. Siladifluoromethylation and deoxo-trifluoromethylation of PV–H compounds with TMSCF3: route to PV–CF2– transfer reagents and P–CF3 compounds. Org. Lett. 21, 1526–1529 (2019).
Anders, E. & Markus, F. Neue methode zur regiospezifischen substitution einiger reaktionsträcer N-heteroaromatischer ringsysteme. Tetrahedr. Lett. 28, 2675–2676 (1987).
Hilton, M. C., Dolewski, R. D. & McNally, A. Selective functionalization of pyridines via heterocyclic phosphonium salts. J. Am. Chem. Soc. 138, 13806–13809 (2016).
Fasano, V., LaFortune, J. H. W., Bayne, J. M., Ingleson, M. J. & Stephan, D. W. Air- and water-stable Lewis acids: synthesis and reactivity of P-trifluoromethyl electrophilic phosphonium cations. Chem. Commun. 54, 662–665 (2018).
Barbe, G. & Charette, A. B. Highly chemoselective metal-free reduction of tertiary amides. J. Am. Chem. Soc. 130, 18–19 (2008).
Fujimoto, H., Kodama, T., Yamanaka, M. & Tobisu, M. Phosphine-catalyzed intermolecular acylfluorination of alkynes via a P(V) intermediate. J. Am. Chem. Soc. 142, 17323–17328 (2020).
Levy, J. N., Alegre-Requena, J. V., Liu, R., Paton, R. S. & McNally, A. Selective halogenation of pyridines using designed phosphine reagents. J. Am. Chem. Soc. 142, 11295–11305 (2020).
Lim, S. & Radosevich, A. T. Round-trip oxidative addition, ligand metathesis, and reductive elimination in a PIII/PV synthetic cycle. J. Am. Chem. Soc. 142, 16188–16193 (2020).
Dolewski, R. D., Fricke, P. J. & McNally, A. Site-selective switching strategies to functionalize polyazines. J. Am. Chem. Soc. 140, 8020–8026 (2018).
Ji, Y. et al. Innate C–H trifluoromethylation of heterocycles. Proc. Natl Acad. Sci. USA 108, 14411–14415 (2011).
Fujiwara, Y. et al. A new reagent for direct difluoromethylation. J. Am. Chem. Soc. 134, 1494–1497 (2012).
Acknowledgements
This work was supported by the National Science Foundation (NSF) under grant number 1753087. We acknowledge the Rocky Mountain Advanced Computing Consortium (RMACC) Summit supercomputer, supported by the NSF (grants ACI-1532235 and ACI-1532236), and the Extreme Science and Engineering Discovery Environment (XSEDE) (allocations TG-CHE180056 and TG-CHE200033). We thank M. J. Gaunt for help in preparing the manuscript. We dedicate this paper to the memory of Robert M. Williams.
Author information
Authors and Affiliations
Contributions
X.Z., K.G.N., C.P. and J.N.L. carried out and analysed the experiments. A.M. directed the project. A.M., R.S.P. and K.G.N. wrote the manuscript. J.V.A.-R. and R.S.P. designed, carried out and analysed the computational experiments.
Corresponding authors
Ethics declarations
Competing interests
A patent application based on the subject matter of this manuscript has been filed with the United States Patent and Trademark Office.
Additional information
Peer review information Nature thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
This file contains Supplementary Sections 1-18, including Supplementary Figures 1-18, Supplementary Tables 1-10 and 1H, 13C, 19F, and 31P Spectra data- see Contents page for details.
Rights and permissions
About this article
Cite this article
Zhang, X., Nottingham, K.G., Patel, C. et al. Phosphorus-mediated sp2–sp3 couplings for C–H fluoroalkylation of azines. Nature 594, 217–222 (2021). https://doi.org/10.1038/s41586-021-03567-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-021-03567-3
This article is cited by
-
Tunable molecular editing of indoles with fluoroalkyl carbenes
Nature Chemistry (2024)
-
Tunably strained metallacycles enable modular differentiation of aza-arene C–H bonds
Nature Communications (2023)
-
Atroposelective hydroarylation of biaryl phosphines directed by phosphorus centres
Nature Communications (2023)
-
Photoinduced copper-catalyzed C–N coupling with trifluoromethylated arenes
Nature Communications (2023)
-
Catalyst control over pentavalent stereocentres
Nature Communications (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.