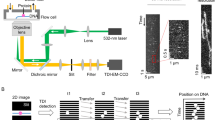
Abstract
Many proteins that bind specific DNA sequences search the genome by combining three-dimensional diffusion with one-dimensional sliding on nonspecific DNA1,2,3,4,5. Here we combine resonance energy transfer and fluorescence correlation measurements to characterize how individual lac repressor (LacI) molecules explore the DNA surface during the one-dimensional phase of target search. To track the rotation of sliding LacI molecules on the microsecond timescale, we use real-time single-molecule confocal laser tracking combined with fluorescence correlation spectroscopy (SMCT–FCS). The fluctuations in fluorescence signal are accurately described by rotation-coupled sliding, in which LacI traverses about 40 base pairs (bp) per revolution. This distance substantially exceeds the 10.5-bp helical pitch of DNA; this suggests that the sliding protein frequently hops out of the DNA groove, which would result in the frequent bypassing of target sequences. We directly observe such bypassing using single-molecule fluorescence resonance energy transfer (smFRET). A combined analysis of the smFRET and SMCT–FCS data shows that LacI hops one or two grooves (10–20 bp) every 200–700 μs. Our data suggest a trade-off between speed and accuracy during sliding: the weak nature of nonspecific protein–DNA interactions underlies operator bypassing, but also speeds up sliding. We anticipate that SMCT–FCS, which monitors rotational diffusion on the microsecond timescale while tracking individual molecules with millisecond resolution, will be applicable to the real-time investigation of many other biological interactions and will effectively extend the accessible time regime for observing these interactions by two orders of magnitude.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
SMCT–FCS raw data are available at https://doi.org/10.17044/scilifelab.12401906. All other data are available from the corresponding authors upon reasonable request. Uncropped gels are provided in Supplementary Fig. 1.
Code availability
Code developed for SMCT–FCS analyses is available at GitHub (https://github.com/elfware/SMCT_FCS). All analysis software developed for this project is available from the corresponding authors upon reasonable request.
References
Berg, O. G., Winter, R. B. & von Hippel, P. H. Diffusion-driven mechanisms of protein translocation on nucleic acids. 1. Models and theory. Biochemistry 20, 6929–6948 (1981).
Hammar, P. et al. The lac repressor displays facilitated diffusion in living cells. Science 336, 1595–1598 (2012).
Blainey, P. C., van Oijen, A. M., Banerjee, A., Verdine, G. L. & Xie, X. S. A base-excision DNA-repair protein finds intrahelical lesion bases by fast sliding in contact with DNA. Proc. Natl Acad. Sci. USA 103, 5752–5757 (2006).
Gowers, D. M., Wilson, G. G. & Halford, S. E. Measurement of the contributions of 1D and 3D pathways to the translocation of a protein along DNA. Proc. Natl Acad. Sci. USA 102, 15883–15888 (2005).
von Hippel, P. H. & Berg, O. G. Facilitated target location in biological systems. J. Biol. Chem. 264, 675–678 (1989).
Blainey, P. C. et al. Nonspecifically bound proteins spin while diffusing along DNA. Nat. Struct. Mol. Biol. 16, 1224–1229 (2009).
Stryer, L. & Haugland, R. P. Energy transfer: a spectroscopic ruler. Proc. Natl Acad. Sci. USA 58, 719–726 (1967).
Ha, T. et al. Probing the interaction between two single molecules: fluorescence resonance energy transfer between a single donor and a single acceptor. Proc. Natl Acad. Sci. USA 93, 6264–6268 (1996).
Bondeson, K., Frostell-Karlsson, A., Fägerstam, L. & Magnusson, G. Lactose repressor-operator DNA interactions: kinetic analysis by a surface plasmon resonance biosensor. Anal. Biochem. 214, 245–251 (1993).
Ha, T., Enderle, T., Chemla, S., Selvin, R. & Weiss, S. Single molecule dynamics studied by polarization modulation. Phys. Rev. Lett. 77, 3979–3982 (1996).
Persson, F., Westerlund, F., Tegenfeldt, J. O. & Kristensen, A. Local conformation of confined DNA studied using emission polarization anisotropy. Small 5, 190–193 (2009).
Backer, A. S., Lee, M. Y. & Moerner, W. E. Enhanced DNA imaging using super-resolution microscopy and simultaneous single-molecule orientation measurements. Optica 3, 659–666 (2016).
Sosa, H., Peterman, E. J., Moerner, W. E. & Goldstein, L. S. ADP-induced rocking of the kinesin motor domain revealed by single-molecule fluorescence polarization microscopy. Nat. Struct. Biol. 8, 540–544 (2001).
Forkey, J. N., Quinlan, M. E., Shaw, M. A., Corrie, J. E. T. & Goldman, Y. E. Three-dimensional structural dynamics of myosin V by single-molecule fluorescence polarization. Nature 422, 399–404 (2003).
Verbrugge, S., Kapitein, L. C. & Peterman, E. J. G. Kinesin moving through the spotlight: single-motor fluorescence microscopy with submillisecond time resolution. Biophys. J. 92, 2536–2545 (2007).
Verbrugge, S., Lansky, Z. & Peterman, E. J. G. Kinesin’s step dissected with single-motor FRET. Proc. Natl Acad. Sci. USA 106, 17741–17746 (2009).
Magde, D., Elson, E. & Webb, W. W. Thermodynamic fluctuations in a reacting system—measurement by fluorescence correlation spectroscopy. Phys. Rev. Lett. 29, 705 (1972).
Persson, F., Lindén, M., Unoson, C. & Elf, J. Extracting intracellular diffusive states and transition rates from single-molecule tracking data. Nat. Methods 10, 265–269 (2013).
Acknowledgements
We thank P. Blainey and K. Xiong for advice on flow-stretching; C. Joo for discussions; J. Laksman for initial code; and D. Fange, I. Barkefors and M. Ehrenberg for helpful comments on the manuscript. The project was funded by the European Research Council (ERC), the Swedish Research Council (VR) and the Knut and Alice Wallenberg Foundation (KAW).
Author information
Authors and Affiliations
Contributions
E.M., B.v.O., G.M. and E.A. contributed equally to this manuscript. S.D. conceived of the smFRET approach to directly observe operator bypassing and J.E. conceived of the confocal tracking with polarization readout concept; S.D., B.v.O., G.M., J.E. and A.S. designed the smFRET study; J.E., E.M. and E.A. conceived of the SMCT–FCS implementation; E.A. developed and built the SMCT–FCS microscope; S.D., E.M. and K.K. developed the purification and labelling scheme; M.J. suggested the use of a bifunctional dye for SMCT–FCS tracking experiments; E.M. and L.C.L. purified and labelled LacI; E.M. implemented the fluidic assay, with input from M.J. E.M. carried out all tracking experiments, with assistance from E.A., K.K. and X.Z.; E.M. and E.A. developed theoretical models and data analysis methods for SMCT–FCS tracking experiments; E.M. carried out analysis of SMCT–FCS tracking experiments; G.M. generated all smFRET DNA constructs and collected smFRET data with B.v.O.; B.v.O. analysed FRET time traces with G.M.; A.E. provided initial code; D.G. supported high-performance liquid chromatography purification and CNBr-mediated cleavage experiments. E.M. designed and carried out stochastic simulations, with input from J.E, S.D. and O.G.B.; S.D., J.E., E.M. and E.A. wrote the paper, with input from all authors.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information Nature thanks Peter von Hippel and Achillefs Kapanidisfor their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data figures and tables
Extended Data Fig. 1 Analysis of LacI labelling and binding.
a, Structural model (based on Protein Data Bank (PDB) code 1OSL). LacI, blue and cyan; rhodamine, green; DNA, grey. b, SDS–PAGE of labelled LacI fractions after dye removal visualized using rhodamine (LacI–R and LacI–MBP–R) or Cy3 (LacI–Far) fluorescence. The bands corresponding to the monomeric sizes expected for LacI–R (left), LacI–Far (middle) and LacI–MBP–R (right) are indicated with arrows. The intensity of the monomeric band relative to the sum of the intensities of monomeric and dimeric bands is 77% and 86% for LacI–R and LacI–MBP–R, respectively. For gel source data, see Supplementary Fig. 1. c, x (DNA direction) and y coordinate of sliding LacI–Far (red) and LacI–R (blue) molecules obtained by electron multiplying charge-coupled device (EMCCD) tracking with a 50-ms frame rate. In total, 779 and 409 sliding molecules were captured for LacI–Far and LacI–R, respectively. Diffusion constants along the x coordinate (Dx) are mean ± s.e.m. d, Mean squared displacement (MSD) for different time steps for LacI–Far (red) (n = 779 sliding molecules) and LacI–R (blue) (n = 409 sliding molecules) ± s.e.m. e, Dissociation constants (Kd) for LacI–Far and LacI–R. LacI = 7.3 nM, NaCl = 1 mM. Kd values were calculated as Kd = koff,obs/kon,obs with errors from propagating errors in the rate constants. Rate constants are mean ± s.e.m. (kon,obs, n = 248 and 200 events for LacI–R and LacI–Far, respectively; koff,obs, n = 367 and 322 events for LacI–R and LacI–Far, respectively).
Extended Data Fig. 2 LacI binding kinetics.
a, EMCCD images of acceptor channel under 532 nm (top) (donor excitation) and 638 nm (bottom) (acceptor excitation) illumination. Specific binding of individual donor-labelled LacI–R molecules (individual spots, top row) occurs in the presence (+O1 DNA; individual spots, bottom row; DNA image was acquired before the addition of LacI, and the same DNA image is shown in columns 3 and 4 for reference) but not in the absence (−O1 DNA) of acceptor-labelled DNA containing an operator site. Addition of isopropyl β-d-1-thiogalactopyranoside (+IPTG) displaces specifically bound LacI in the same field of view. This control experiment was carried out once. b, Time trace of LacI–R occupancy in one field of view (1,244 individual O1 DNA molecules). The 7.3 nM LacI–R was supplied after 20 s (first dotted line), followed by the addition of 7.3 nM LacI–R together with 100 mM IPTG after 90 s. c, Histograms of twait values for specific binding to O1 (n = 310 or 100 trajectories for LacI = 7.3 nM and NaCl = 1 mM or otherwise, respectively) at various LacI and NaCl concentrations as indicated. d, Left, histograms of tdwell values for specific binding to O1 (n = 190 or 184 events at LacI–R = 7.3 nM and NaCl = 1 mM (top) or 80 mM (bottom), respectively). Right, under standard imaging conditions (marked by the asterisk) measurements of tdwell for binding to O1 are not affected by photobleaching. Mean relative tdwell for specific binding to O1 (n = 106, 146, 92 and 243 individual molecules from left to right, with mean binding duration ± s.e.m.) observed at NaCl = 1 mM, and using various laser power densities, is shown. Dwell times and laser power densities were normalized to the standard imaging laser power density used for all other analyses (asterisk). e, Histogram of twait values for specific binding to O3 (n = 109 individual molecules) at LacI = 7.3 nM and NaCl = 1 mM. f, Left, histogram of tdwell values for specific binding to O3 (n = 91 individual molecules at LacI–R = 7.3 nM and NaCl = 1 mM). Right, under standard imaging conditions (marked by the asterisk), measurements of tdwell for binding to O3 are not affected by photobleaching. The mean relative tdwell for specific binding to O3 (n = 79, 196, 83 and 107 individual molecules from left to right, with mean binding duration ± s.e.m.) observed at NaCl = 1 mM, and using various laser power densities, is shown. Dwell times and laser power densities were normalized to the standard imaging laser power density used for all other analyses (asterisk). g, Dependence of the mean twait (left) and tdwell (right) value for binding on NaCl (LacI–R = 7.3 nM) concentrations. twait (left), n = 130 or 100 individual molecules for NaCl = 1 mM or for all other conditions, respectively; tdwell (right), n = 190, 164, 164, 82, 184 and 153 individual molecules from left to right. Data are shown as mean ± s.e.m.
Extended Data Fig. 3 Predominant switching and flipping transitions.
Left, cartoon schematic of the six distinct LacI–R binding states (Supplementary Methods) observed with a construct featuring two outer O1 sites. Transitions between distinct states are depicted by arrows. Right, switching rates observed at various LacI–R concentrations, normalized to the LacI–R concentration of 0.9 nM, (asterisk, used for the determination of all switching rates shown in Fig. 2), which is in the concentration regime in which switching is not affected by the occupancy of multiple LacI molecules. Error estimates represent s.e.m. (n = 22,720, 12,408, 13,714 and 3,601 individual molecules from left to right).
Extended Data Fig. 4 Camera-based polarization measurements and characterization of dye labelling.
a, SDS–PAGE analysis of LacI–R and LacI–Cy3-2 after peptide cleavage with CNBr. LacI–R was designed for bifunctional labelling (rhodamine attachment to both adjacent Cys residues) and LacI–Cy3-2 for monofunctional (using only one of the two Cys) labelling of the same α-helix. Precision Plus Protein Dual Xtra Standards (Bio-Rad) were used as a ladder for both gels. The experiments were repeated at least three times. For gel source data, see Supplementary Fig. 1. b, Schematic of the setup for camera-based polarization measurements. c, P0 (blue) and P45 (red) polarization distributions averaged over 600 ms (3 frames) per count measured for sliding LacI–R (left) (79 and 172 sliding events for P0 and P45, respectively) and LacI-Cy3 (right) (61 and 53 sliding events for P0 and P45, respectively). For P0 measurements, the horizontal polarization axis was aligned with the stretching direction of DNA; for P45 measurements, the horizontal polarization axis was rotated 45° away from the stretching direction of DNA. Details are provided in Supplementary Methods. d–f, Simulated polarization distributions for the uniform (d), linear (e) and rotation-coupled (f) sliding models. g, h, Mean ± s.e.m. (g) and s.d. ± s.e. (h) of the experimental polarization distributions in c, j. From left to right, n = 800, 1,606, 887, 673, 78 and 104 polarization signals averaged over 600-ms time steps. Error bars represent s.e.m. i, Schematic of the 7-kb DNA used in operator-binding polarization measurements (top) and EMCCD image of LacI–R bound to the artificial, strong Osym operator on the flow-stretched DNA, in which 11 binding events were detected for measurements of P0 (bottom). j, P0 (blue) and P45 (red) polarization distributions averaged over 600 ms (3 frames) per count measured for operator-bound LacI–R. Eleven and 15 binding events were detected for measurements of P0 and P45, respectively.
Extended Data Fig. 5 Optical layout and calibration data for SMCT–FCS.
a, Optical layout of the combined system for fluorescence polarization microscopy and single-molecule confocal tracking. In the excitation path, a half-wave plate (HWP) and a quarter-wave plate (QWP) are placed behind a polarizer to create circularly polarized light at the objective. APD, avalanche photodiode; DM, dichroic mirror; HWP, half-wave plate; PBS, polarization beam splitter; QWP, quarter-wave plate. b, Immobilized fluorescent beads were used as a platform for testing the tracking capabilities of the real-time tracking system. A tracking trajectory is shown in which the stage is moving in circles with a diameter of 2.8 μm and the photon count rate is on average 24 kc s−1 (18 kc s−1 including laser off-time). Each greyed-out area represents a 1-s revolution followed by an 800-ms pause. In each paused section, the positioning s.d. is 20 nm and includes stage noise from its feedback loop. c, ACF for the bead shown in b. The plot depicts the high afterpulsing peak at 20 ns, which rapidly decreases and flattens out after 500 ns. The grey area corresponds to the time regime plotted for ACFs in Fig. 3. After 1 ms, an oscillation appears which is caused by the 4-ms instrument tracking period. The autocorrelation curve is here compensated for the 166-μs measurement off time between each 500-μs measurement.
Extended Data Fig. 6 Traces captured with confocal tracking.
a, Left, x (DNA direction) and y coordinate of sliding LacI–MBP–R (blue) and LacI–R (red) molecules obtained by confocal tracking. In total, 151 and 90 sliding molecules were captured for LacI–MBP–R and LacI–R, respectively. The average diffusion constants along the x coordinate were 0.027 ± 0.001 μm2 s−1 and 0.035 ± 0.002 μm2 s−1. Diffusion constants are indicated as 20% trimmed mean ± s.e.m. Right, mean squared displacement for different time steps for LacI–MBP–R (blue) (n = 151 sliding molecules) and LacI–R (red) (n = 90 sliding molecules) ± s.e.m. b, x (DNA direction) and y coordinate of stationary LacI–MBP–R (blue) and LacI–R (red) molecules obtained by confocal tracking. For clarity, 100 representative trajectories are shown for each LacI species. In total, 3,773 and 1,594 stationary molecules were detected for LacI–MBP–R and LacI–R, respectively.
Extended Data Fig. 7 Fit of pitch-dependent autocorrelation model and repeats of SMCT–FCS experiments.
a, Pitch estimates from simulated autocorrelations as a function of the pitch used in the simulation for different amplitudes of the background. b, Error in the pitch estimate as a function of background amplitude for different pitches. To consider only the simulated results relevant for the experimental results observed in Fig. 3, values are reported only for the estimated pitches between 9 and 75 bp and when the largest distance (amplitude) of the difference in the simulated autocorrelation is at least 50% of the amplitude of the observed experimental difference. c, The average normalized autocorrelation of the fluorescence signal for sliding (left) and stationary (right) LacI–MBP–R (blue) and LacI–R (red) molecules when the data have been pooled in two series (separated chronologically when the data were captured) for earlier (top) and later (bottom) recorded data. The reported diffusion constants are averages of all tracked molecules for each series. Error bars and error estimates represent s.e. Diffusion constants are indicated as 20% trimmed mean ± s.e.m. For the first experimental series, 69 sliding and 2,758 stationary molecules were analysed for LacI–MBP–R, and 36 sliding and 903 stationary molecules were analysed for LacI–R. For the second experimental series, 82 sliding and 1,015 stationary molecules were analysed for LacI–MBP–R, and 54 sliding and 691 stationary molecules were analysed for LacI–R. Middle, the difference in the mean autocorrelation between LacI–MBP–R and LacI–R ± s.e. Lines represent the best fit to a rotation-coupled sliding model. d, Difference in autocorrelation from simulations replicating the experimental datasets and filtering steps. The autocorrelation differences are indicated by the red dots. The number of traces, average trace length and average photon counts were the same as in the experiments, and the rotational diffusion constants were the fitted values from the experiments. The mean and expected s.e. (blue crosses) was calculated as the mean and s.d. of 60 individual simulations. The mean and s.e. of the experiments (black diamonds) were estimated as described in Fig. 3g (n = 86 and 54 informative autocorrelations for LacI–MBP–R and LacI–R, respectively).
Extended Data Fig. 8 Probability densities of model parameters when taking into account the experimental FRET results.
Model parameters from simulations that are compatible with the observed rates of switching between two outer O1 operators with (blue) and without (purple) an intervening internal O1 operator, and compatible with the flipping orientation on the same operator (orange). The transparency values of the surfaces have been scaled with the probability density for the parameters given the experimental data. The mean and standard error of the average hop length calculated from the joint probability densities for the four different kon,μ values are—from left to right—22 ± 16 bp, 18 ± 11 bp, 16 ± 8 bp and 18 ± 12 bp. The flipping rate surface does not decrease with increasing values of koff,μ, as the simulations yield the maximum possible flipping rate. In other words, parameters that correspond to a higher maximum flipping rate could have also generated a lower actual flipping rate. Although kon,μ was finite and fixed at four different levels in these simulations, kon,μ was infinite in the simulations shown and used in Fig. 4c–e. The sample size was n = 1,108, 574 and 699 individual molecules for the O1-random-O1 switching rate, the LacI–R flipping rate and the O1-O1-O1 switching rate, respectively.
Supplementary information
Supplementary Information
This file contains: Supplementary Methods, Theory and a Detailed Analysis and Discussion; Supplementary Figures 1-4; Supplementary Tables 1-3 and Supplementary References.
Rights and permissions
About this article
Cite this article
Marklund, E., van Oosten, B., Mao, G. et al. DNA surface exploration and operator bypassing during target search. Nature 583, 858–861 (2020). https://doi.org/10.1038/s41586-020-2413-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2413-7
This article is cited by
-
Dynamic interplay between target search and recognition for a Type I CRISPR-Cas system
Nature Communications (2023)
-
Real-time single-molecule 3D tracking in E. coli based on cross-entropy minimization
Nature Communications (2023)
-
High-throughput biochemistry in RNA sequence space: predicting structure and function
Nature Reviews Genetics (2023)
-
Spatiotemporally controlled generation of NTPs for single-molecule studies
Nature Chemical Biology (2022)
-
Sequence-dependent surface condensation of a pioneer transcription factor on DNA
Nature Physics (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.